9.5.2 System and Surrounding
This is the second lecture from Chapter 5: “Energetics” in the new Class 9 Chemistry book (Punjab Board – PCTB). It discusses the concepts of system and surrounding. The lecture also includes a multiple-choice quiz, short question and long question notes.
MCQs Based Quiz
Short Questions
Q1. What is a system?
In chemistry, any physical and chemical change under study is called a system.
Q2. What does a chemical reaction include?
A chemical reaction includes reactants, products, catalysts, solvents and any other matter that is important to study that chemical reaction.
Q3. What is meant by surrounding in terms of thermodynamics?
Everything else which is not the part of system is called surrounding.
Q4. How would you explain the terms of system and surrounding with the help of a simple example?
If you are studying the boiling of water in a beaker, the water molecules will be called system, while everything else like beaker, burner, etc., will be called the surrounding.
Q5. How would you define an endothermic energy change?
If energy is transferred from surrounding to the system, the change will be called an endothermic change and it will have a positive sign.
Q6. What is meant by exothermic energy change?
If energy is transferred from system to surrounding, the change will be called an exothermic change and it will have a negative sign.
Q7. How can you use the energy evolved during a chemical reaction?
This evolved energy is used in everyday life for cooking, heating, lighting, transportation and much more.
Q8. Is boiling water in a beaker an endothermic change or an exothermic change? Which form of energy is being transferred in this system?
When was water is boiled in a beaker, the energy is transferred from surrounding (burner) to the system (water molecules). Therefore, it will be called an endothermic change. During this process, the heat energy is transferred from burner to water molecules.
Q9. What are the differences between endothermic and exothermic processes?
Endothermic Processes
Exothermic Processes
In an endothermic process, the system absorbs energy from the surrounding.
In an exothermic process, the system releases energy into the surrounding.
In this process the system gains energy (heat).
In this process the system loses energy (heat).
In this process surroundings get colder.
In this process surroundings get warmer.
The energy change in this process has positive sign (+).
The energy change in this process has negative sign (-).
Example: Melting of ice
Example: Burning of fuel
Descriptive Question
Q1. Write a comprehensive note on system and surrounding.
System: In chemistry, any physical or chemical change that is under study is called a system.
Surrounding: Everything else which is not a part of system is called surrounding.
Chemical Reaction: A chemical reaction or chemical change includes reactants, products, catalysts, solvents and any other matter that is important to study that chemical reaction.
Example: If you are studying the boiling of water in a beaker, the water molecules will be called system, while everything else like beaker, burner, etc. will be called the surrounding.
Energy transfer between system and surrounding:
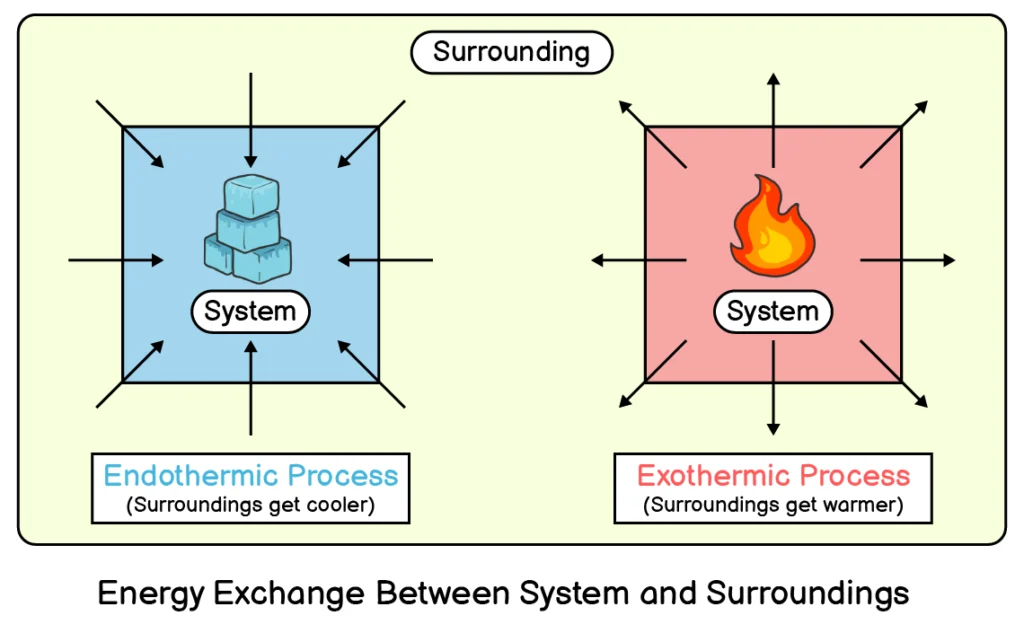
Endothermic Change: If energy is transferred from surrounding to the system, the change will be called an endothermic change, and it will have a positive sign.
Exothermic Change: If energy is transferred from system to surrounding, the change will be called an exothermic change, and it will have a negative sign.
Example: When water is boiling in a beaker, the energy is transferred from surrounding (burner) to the system (water molecules). Therefore, it will be called an endothermic change. During this process, the heat energy is transferred from burner to water molecules.
Similarly the exothermic changes are also used in our everyday life for cooking, heating, lighting, transportation and much more.