9.5.5 How does a Reaction Take Place
This is the fifth lecture from Chapter 5: “Energetics” in the new Class 9 Chemistry book (Punjab Board – PCTB). It discusses the basic concepts involved in chemical reactions, such as reactant collisions, transition state, activation energy, and energy profile diagrams. The lecture also includes a multiple-choice quiz, short question and long question notes.
MCQs Based Quiz
Short Questions
Q1. What is a transition state?
A high energy state that is formed due to collision between reactant molecules is called transition state.
Q2. Why do reactant molecules have kinetic energy?
Reactant molecules in a container have constant random motion colliding with each other and the walls of container. This constant motion gives reactant molecules their kinetic energy.
Q3. Do all the reactant molecules have exactly equal amounts of kinetic energy?
In a reaction mixture, there are three types of reactant molecules:
- Those with average kinetic energy
- Those with less than average kinetic energy
- Those with more than average kinetic energy
Q4. What are excited reactant molecules?
Reactant molecules that have more than average kinetic energy are called excited reactant molecules.
Q5. What are the possible outcomes of the collision between reactant molecules?
- The collision between reactant molecules with average or less than average kinetic energy may not produce any results.
- The collision between reactant molecules with more than average kinetic energy (excited molecules) will produce transition state.
Q6. How is a transition state formed in a chemical reaction?
A transition state is formed due to a collision between excited reactant molecules. It is a state that is intermediate between both reactants and products.
Q7. What happens to the transition state after the reaction proceeds?
- The transition state can either return to reactants.
- Or it can convert into products.
Q8. Draw an energy diagram for an exothermic reaction.
Q9. Draw an energy diagram for an endothermic reaction.
Q10. Why is the energy of the transition state higher than that of the reactants or products?
Energy of the transition state is higher than that of the reactants and products because, in this state, old bonds are being broken while new bonds are not yet formed properly.
Q11. How can you determine whether a reaction is endothermic or exothermic by comparing the energies of reactants and products?
- If reactants have higher energy than that of products, the reaction will be exothermic.
- If the reactants have lower energy than that of products, the reaction will endothermic.
Q12. How would you define activation energy (Ea)of the reaction?
The amount of energy absorbed by reactants or products to be converted into transition state is called the activation energy (Ea) of the reaction.
Q13. Are energy diagrams useful?
Yes, they are useful because:
- They help determine whether a reaction is exothermic or endothermic
- They indicate the activation energy of the reaction.
- They also help us study the role of catalysts
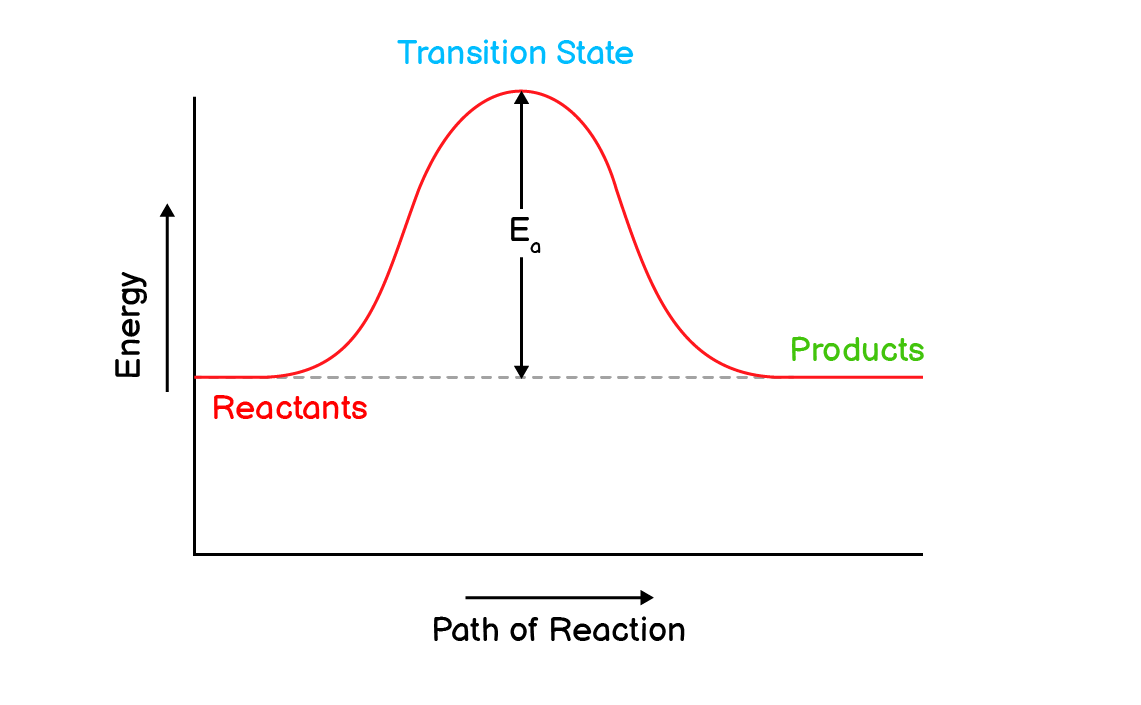
Q14. Draw an energy profile diagram for a hypothetical reaction which does not evolve or absorb heat.
A reaction that does not evolve or absorb heat (energy) will have equal energies of reactants and products.
Q15. What is a catalyst?
A catalyst is a substance that can increase the rate of reaction without undergoing any permanent chemical change.
For Example: Nickel acts as a catalyst in the hydrogenation of oil to give Banaspati Ghee.
Q16.How does a catalyst increase the rate of reaction?
A catalyst increases the rate of reaction by adopting a path of reaction with much less activation energy so that reactants can easily convert into products.
Descriptive Questions
Q1. How does the collision of reactant molecules result in the formation of products?
Steps in a Chemical Reaction:
A chemical reaction takes place through the following steps:
- Collision of reactant molecules to produce transition state
- Conversion of transition state into the products
To understand this, let’s study this hypothetical reaction:
$\mathrm{
A_2 + B_2 \longrightarrow 2AB
}$
Collision of Reactant Molecules:
Before mixing reactants A and B:
- Reactant A and B are in separate containers.
- Their molecules are in state of random motion.
- They collide with each other and with the walls of container.
- Majority of the molecules have average kinetic energy.
- Few of them have more than average kinetic energy.
- Some of them have less than average kinetic energy.
- Molecules with more than average kinetic energy are called excited reactant molecules.
After mixing reactants A and B:
- Molecules of all the reactants start colliding with each other.
- The collision between reactant molecules with average or less than average kinetic energy may not produce any results.
- The collision between reactant molecules with more than average kinetic energy (excited molecules) may produce transition state.
Transition State and the Formation of Products:
- The energy of transition state is higher than that of reactants or products.
- This is because in this state, old bonds are being broken while new bonds are not yet formed properly.
- This transition state can either return to the reactants, resulting in the failure of the reaction.
- Or it can convert into products which successfully completes a reaction.
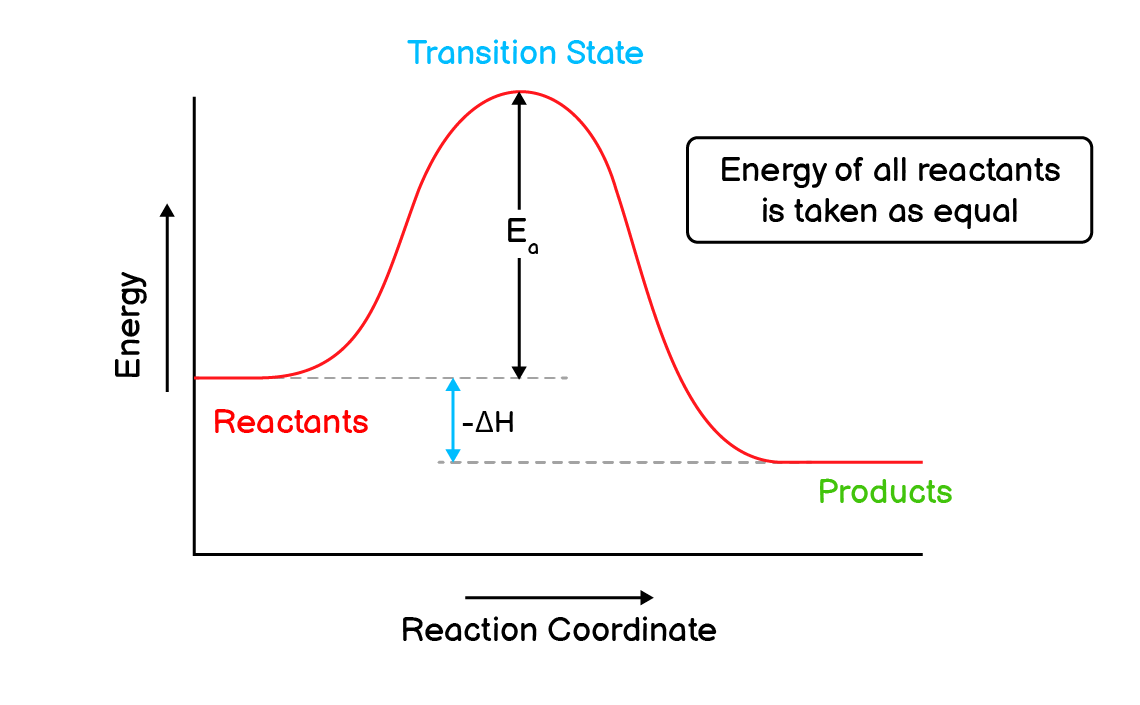
Q2. Explain a hypothetical exothermic reaction with the help of energy profile diagram.
Exothermic Reactions:
A type of reaction that involves the release (evolution) of energy (heat) is called an endothermic reaction.
Consider a hypothetical exothermic reaction that releases ‘E’ amount of energy,
$\mathrm{
A_2 + B_2 \longrightarrow 2AB + E
}$
The energy profile diagram for this reaction will be as follows:
According to this diagram:
- The reaction starts with the absorption of activation energy $\mathrm{(E_a)}$ by reactants.
- After the absorption of activation energy, the reactants form a transition state.
- The transition state has the highest energy compared to reactants and products.
- This transition state can either return to the reactants, resulting in the failure of the reaction.
- Or it can convert into products which successfully completes a reaction.
- Because this is an exothermic reaction, the energy of product will always be less than that of reactants.
- This difference of energy in reactants and products is equal to change in enthalpy $\mathrm{\Delta H}$.
- The negative sign with the enthalpy indicates that this is an exothermic reaction.
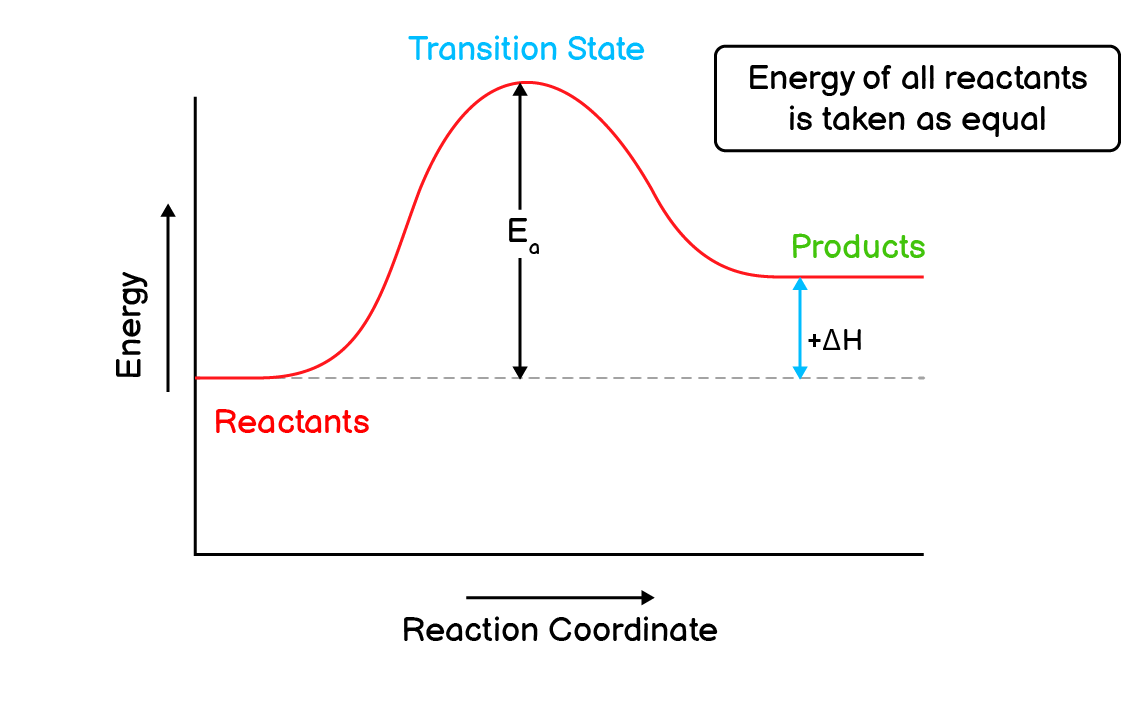
Q3. Explain a hypothetical endothermic reaction with the help of energy profile diagram.
Endothermic Reactions:
A type of reaction that involves the absorption of energy (heat) is called an endothermic reaction.
Consider a hypothetical exothermic reaction that absorbs ‘E’ amount of energy,
$\mathrm{
A_2 + B_2 \longrightarrow 2AB – E
}$
The energy profile diagram for this reaction will be as follows:
According to this diagram:
- The reaction starts with the absorption of activation energy $\mathrm{(E_a)}$ by reactants.
- After the absorption of activation energy, the reactants form a transition state.
- The transition state has the highest energy compared to reactants and products.
- This transition state can either return to the reactants, resulting in the failure of the reaction.
- Or it can convert into products which successfully completes a reaction.
- Because this is an endothermic reaction, the energy of product will always be more than that of reactants.
- This difference of energy in reactants and products is equal to change in enthalpy $\mathrm{\Delta H}$.
- The positive sign with the enthalpy change indicates that this is an exothermic reaction.
Q4. What is a catalyst? How does it affect the rate of a chemical reaction? Explain with the help of energy profile diagram.
A catalyst is a substance that can increase the rate of reaction without undergoing any permanent chemical change.
A catalyst increases the rate of reaction by adopting a path of reaction with much less activation energy so that reactants can easily convert into products. As a result, more reactants are now able to be converted into products and hence the speed of reaction increases.
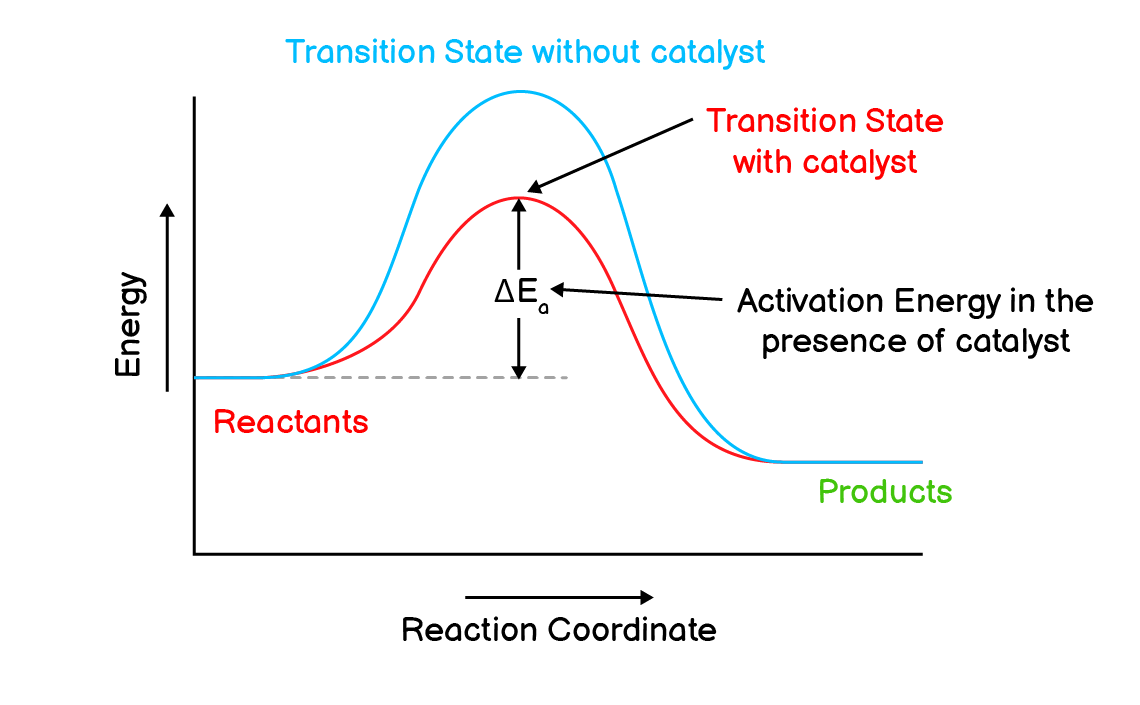
Examples:
- Nickel (Ni) acts as a catalyst in the hydrogenation of oil to give Banaspati Ghee.
- Platinum (Pt) acts as a catalyst in the production of sulphuric acid $\mathrm{(H_2SO_4)}$.
- Chlorine acts as a catalyst in the breakdown of ozone.