Chapter 5: Energetics (Solved Exercise Notes)
This is solved exercise notes for chapter 5: ‘Energetics’ of the new book for Class 9 Chemistry Punjab Board (PCTB) 2025. These notes include solved multiple choice questions (MCQs), questions for short answers, constructed response questions, descriptive questions and investigative questions.
1. Multiple Choice Questions
1. The following reaction is an exothermic reaction.
$\mathrm{H_2\ +\ Cl_2\ \xrightarrow[]{Sunlight} \ 2HCl}$
From where does the energy come to break the bonds of $\mathrm{H_2}$ and $\mathrm{Cl_2}$?
(a) By collisions between the molecules
(b) From sunlight
(c) From the surrounding
(d) By collisions of the molecules with the walls of the container
2. Which of the following reactions has the least value of activation energy?
(a) $\mathrm{ H_{2(g)}\ +\ \frac{1}{2} O_{2(g)}\ \longrightarrow \ H_2O_{(g)} }$
(b) $\mathrm{ C_{(s)}\ +\ O_{2(g)}\ \longrightarrow \ CO_{2(g)} }$
(c) $\mathrm{ NaCl_{(aq)}\ +\ AgNO_{3(aq)} \longrightarrow \ AgCl_{(s)}\ +\ NaNO_{3(aq)} }$
(d) $\mathrm{ H_{2(g)}\ +\ I_{2(g)}\ \longrightarrow \ 2HI_{(g)} }$
3. Formation of which hydrogen halide from the elements is an endothermic reaction?
(a) $\mathrm{HCl}$
(b) $\mathrm{HF}$
(c) $\mathrm{HBr}$
(d) $\mathrm{HI}$
4. What are the products of anaerobic respiration?
(a) ATP + CO2 + H2O
(b) CO2 + H2O
(c) ATP + Ethanol + CO2
(d) Ethanol + H2O
(Correction: In the book, none of these options are correct, so H2O in option C was replaced with CO2)
5. Which reaction do you expect to be a reversible reaction?
(a)
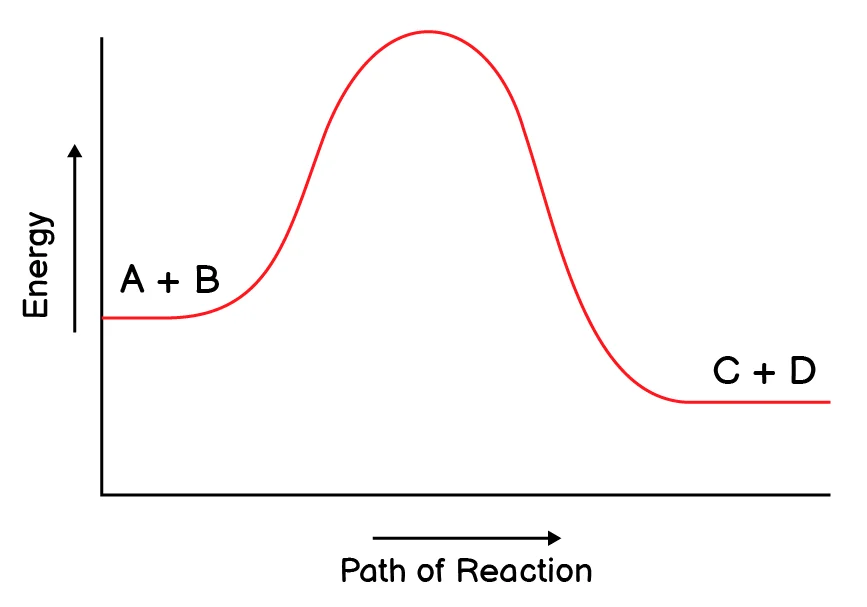
(b) Correct
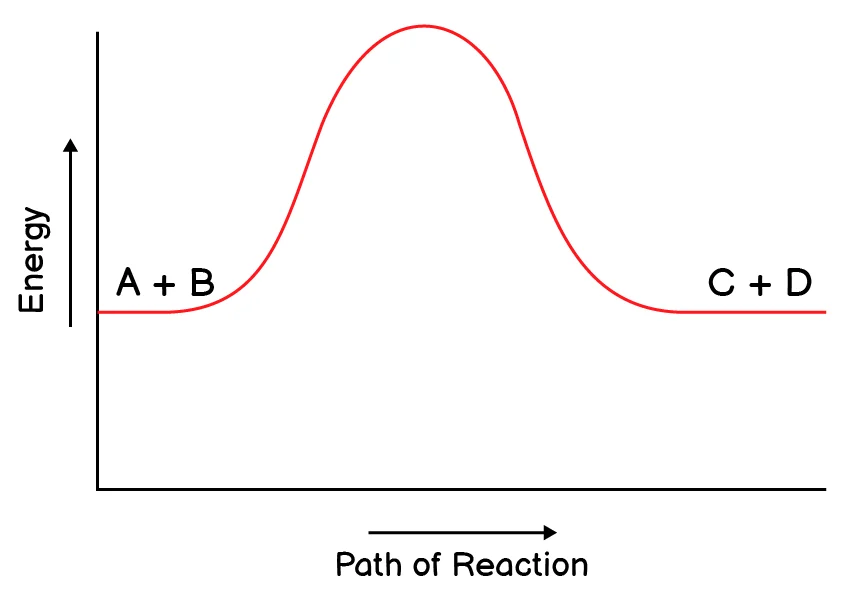
(c)
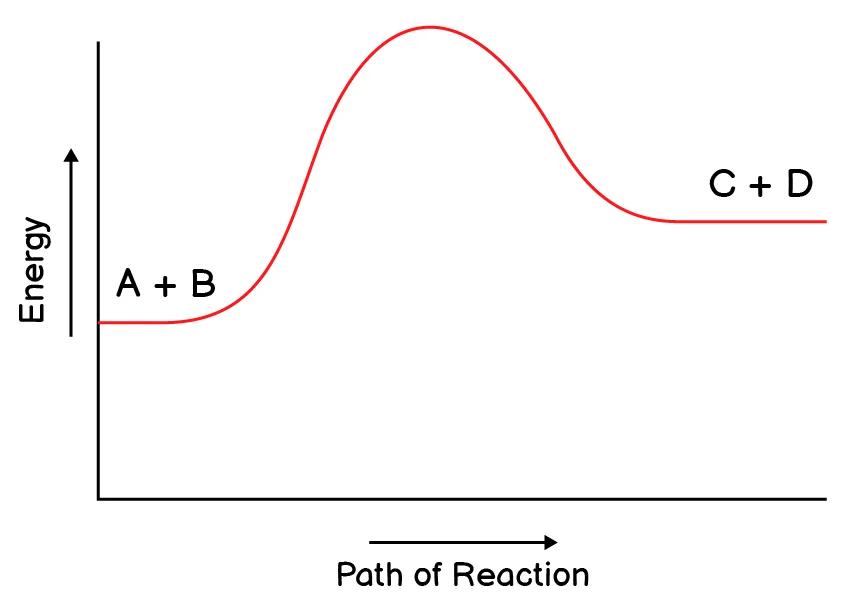
(d)
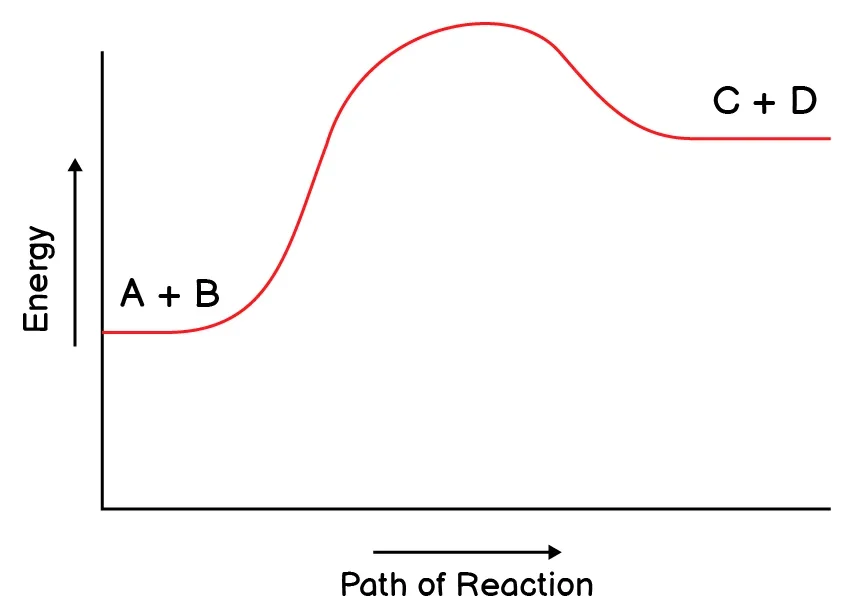
6. What does it show when a chemical reaction is exothermic?
(a) It shows the bonds which break are weaker than those are formed.
(b) It shows the bond which break are stronger than those are formed.
(c) Exothermic nature of the reaction is not concerned with bond formation or bond breakage.
(d) It shows that the reactants are more stable than the products.
7. When NaOH and HCl are mixed the temperature increases. The reaction is:
(a) endothermic with a positive enthalpy change.
(b) endothermic with a negative enthalpy change.
(c) exothermic with a positive enthalpy change.
(d) exothermic with a negative enthalpy change.
8. The average bond dissociation energy for the C-H bond is $\mathrm{412\ kJ\ mol^{-1}}$. Which of the following process will have enthalpy change close to $\mathrm{412\ kJ\ mol^{-1}}$?
(a) $\mathrm{ CH_{4(g)}\ \longrightarrow \ C_{(g)}\ +\ 2H_{2(g)} }$
(b) $\mathrm{ CH_{4(g)}\ \longrightarrow \ C_{(g)}\ +\ 2H_{2(g)} }$
(c) $\mathrm{ CH_{4(g)}\ \longrightarrow \ C_{(g)}\ +\ 4H_{(g)} }$
(d) $\mathrm{ CH_{4(g)}\ \longrightarrow \ CH_{3(g)}\ +\ H_{(g)} }$
9. The average bond energies for O-O and O=O are $145$ and $\mathrm{496\ kJ\ mol^{-1}}$ respectively. Find the enthalpy in kJ for the following reaction.
$\mathrm{H-O-O-H_{(g)} \longrightarrow\ H-O-H_{(g)}\ +\ \frac{1}{2}\ O=O_{(g)}}$
(a) $\mathrm{-102kJ}$
(b) $\mathrm{+102kJ}$
(c) $\mathrm{+350kJ}$
(d) $\mathrm{+394kJ}$
10. Why does the following exothermic reaction not occur?
$\mathrm{C(Diamond) \longrightarrow\ C(Graphite)\ \quad\ \boxed{ \Delta \mathrm{H=-3kJ\ mol^{-1}}}}$
(a) Structure of diamond is more stable than that of graphite.
(b) Diamond has strong covalent bonds than does the graphite.
(c) The change from diamond to graphite has high activation energy.
(d) Density of graphite is less then that of diamond.
2. Questions for Short Answers
Q1. What is the difference between enthalpy and enthalpy change?
Enthalpy
Enthalpy Change
Total amount of thermal energy stored in a system is called enthalpy $\text{H}$.
Heat absorbed or released by a system at constant pressure is called change in enthalpy $\mathrm{\Delta H}$ of that system.
It is an essential part of the system.
It is not an essential part of the system.
It can not be measured directly.
It can be measured directly.
Its unit is kJ/mol.
Its unit is also kJ/mol.
Q2. Why is breaking of a bond an endothermic process?
A chemical bond is an attractive force between two atoms. Breaking of this attractive force requires absorption of energy which makes it an endothermic process.
Q3. Depict the transition state for the following reaction:
$\mathrm{ H_{2(g)}\ +\ Cl_{2(g)}\ \longrightarrow \ 2HCl_{(g)} }$
The following is the transition state of the reaction.
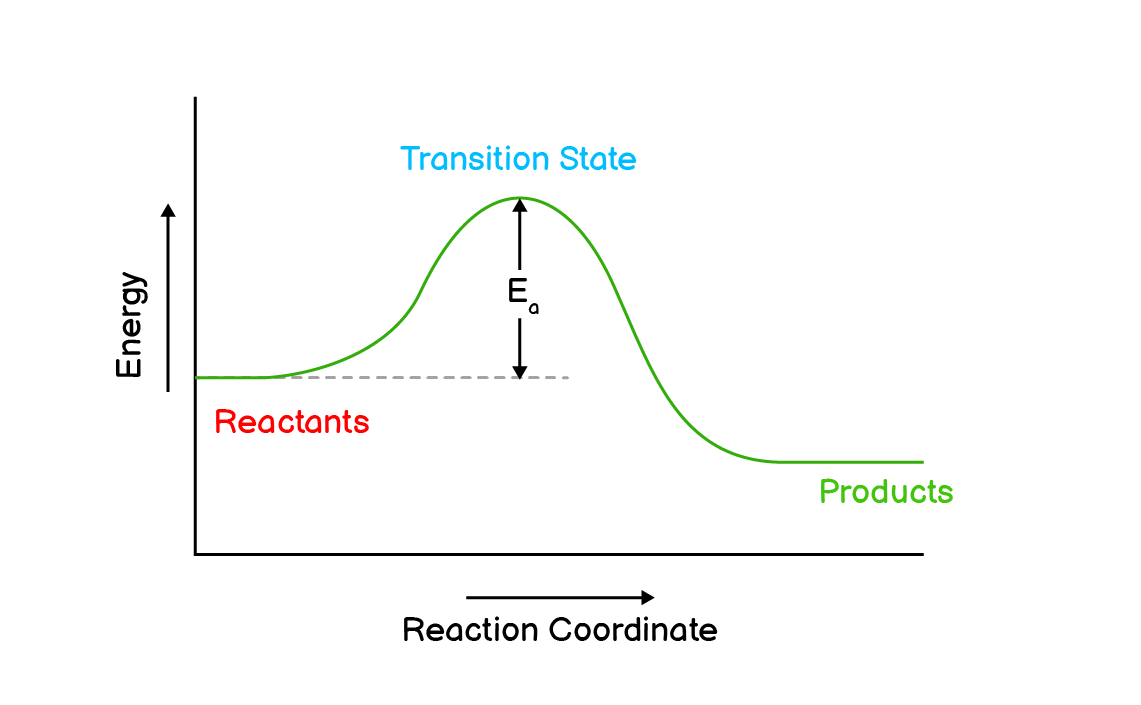
Q4. Draw the reaction profiles for two exothermic reactions, one of which moves faster than the other.
(a). This reaction has higher activation energy so it moves slower than the other.
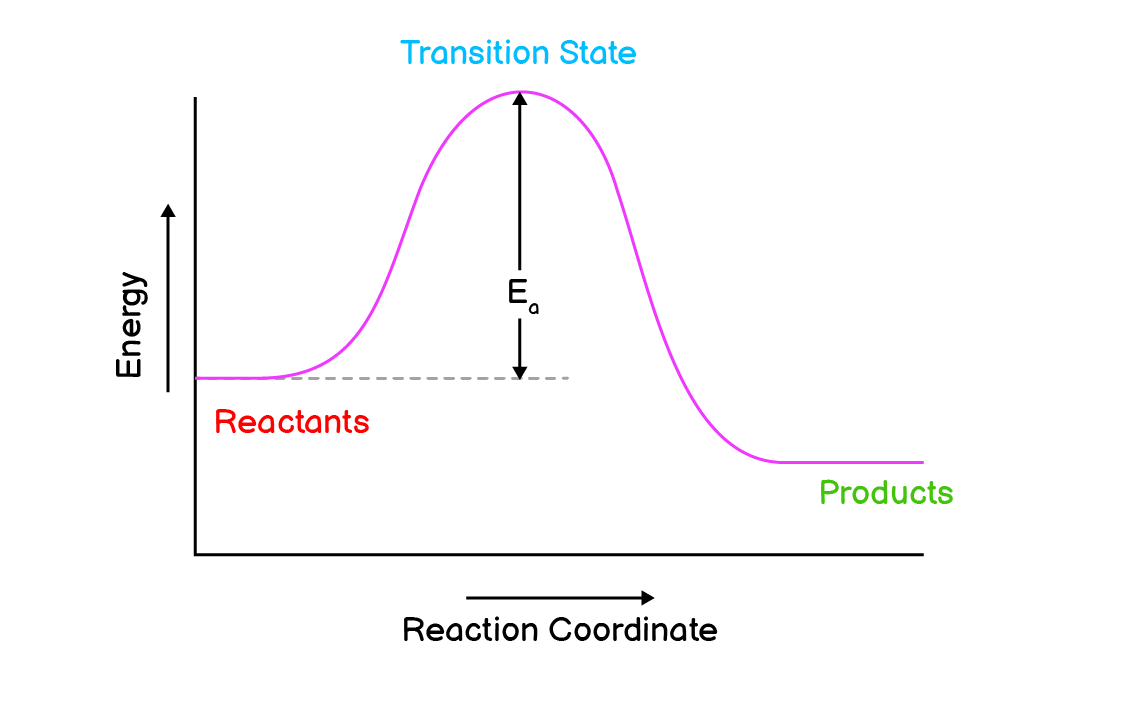
(b). This reaction has lower activation energy so it moves faster than the first one.
Q5. What is the role of glycogen in our body?
Glycogen is primary storage form of glucose in our body. It is stored in liver and muscles. Its role is to maintain blood glucose level and give immediate energy to muscles.
3. Constructed Response Questions
Q1. Physical changes which usually occur around us are given in the table. Write down whether they are exothermic or endothermic.
Physical Change
Exothermic/Endothermic
Conversion of hydrated salt into anhydrous salt
Endothermic
Conduction of electricity by metals
Exothermic
Burning of paper
Exothermic
Dissolving ammonium chloride in water
Endothermic
Vapourizing liquid nitrogen
Endothermic
Formation of rain from clouds
Exothermic
Evaporation of dry ice
Endothermic
Dissolving sodium carbonate in water
Exothermic
Q2. Explain why the reaction between atmospheric gases oxygen and nitrogen does not take place under normal conditions? But in the presence of lightning these gases react to give NO. The reaction stops as soon as the lightning stops.
$\ce{N_2 + O_2 \longrightarrow 2NO}$
The reaction between atmospheric nitrogen and oxygen is an endothermic reaction, which requires a constant supply of energy provided by lightning. As soon as the lightning stops, the supply of energy is cut off, and the reaction stops as well.
$\ce{N_2 + O_2 \longrightarrow 2NO – 180.6 kJ}$
Q3. A reaction between natural gas (CH4) and atmospheric oxygen does not take place when you mix them. As soon as you show a burning match stick, the reaction starts immediately and then it continues until one or both of the reactants is/are used up. Explain.
The reaction between natural gas ($\ce{CH_4}$) and oxygen ($\ce{O_2}$) is exothermic but needs activation energy to start. This energy breaks the bonds in reactants. Once started, the heat released keeps the reaction going by breaking more bonds.
4. Descriptive Questions
Q1. Find out the enthalpy change of the following reaction using the given data.
$\ce{N_2 + O_2 \longrightarrow 2NO}$
Bond dissociation energy (BDE) of $\ce{N_2}$ = 958.38 kJ/mol
Bond dissociation energy (BDE) of $\ce{O_2}$ = 498 kJ/mol
Bond formation energy (BFE) of $\ce{NO}$ = -626 kJ/mol
Solution:
$$\begin{aligned}
\mathrm{Total\ BDE\ (Endothermic)} &= \mathrm{BDE\ of\ } \ce{N2} + \mathrm{BDE\ of\ } \ce{O2} \\
&= 958.38 + 498 \\
&= 1456.38\ \mathrm{kJ/mol} \\
\\
\mathrm{Total\ BFE\ (Exothermic)} &= \mathrm{BFE\ of\ } \ce{NO} \times \mathrm{No.\ of\ moles\ of\ } \ce{NO} \\
&= -626 \times 2 \\
&= -1252\ \mathrm{kJ/mol} \\
\\
\Delta\mathrm{H} &= \mathrm{Total\ BDE} + \mathrm{Total\ BFE} \\
&= 1456.38 + (-1252) \\
&= 204.38\ \mathrm{kJ/mol}
\end{aligned}$$
Because change in enthalpy ($\ce{\Delta \ce{H}}$) is positive, this reaction is endothermic.
Q2. Explain the difference between the terms heat and enthalpy.
Heat
Enthalpy
Heat it a form of energy that flows from a hot system to a cold system due to difference in temperature.
The total amount of thermal energy stored in a system is called the enthalpy of that system.
Heat is represented by ‘q’.
Enthalpy is represented by ‘H’.
It is not an essential part of the system.
It is an essential part of the system.
A system can gain or lose heat.
Enthalpy change is equal to change in heat at constant pressure.
It is measured in joules (J).
It is measured in kJ mol-1.
Q3. Explain why formation of a bond is always an exothermic process.
Formation of chemical bonds between two atoms is always an exothermic process meaning that chemical bond formation will always release energy. This is because atoms in unbonded state are chemically unstable. Chemical instability means that they are in higher energy state.
When a chemical bond is formed between two atoms, it makes them stable lowering their energy. Therefore, during a chemical bond formation, atoms release their excess energy and achieve stable or lower energy state. This makes chemical bond formation an exothermic process.
$\mathrm{
\underset{\color{red}(Unstable/High\ Energy)}{O_{(g)}\ +\ 2H_{(g)}} \longrightarrow \underset{\color{green}(Stable/Low\ Energy)}{H_2O_{(g)}}\ +\ 284kJ/mol
}$
Q4. Explain the role of lipids in our body.
Lipids in our body have the following roles:
- Lipids are group of organic compounds which include fats, waxes, sterols, etc.
- Lipids serve as energy reserve in our bodies.
- They fulfil almost half of the energy needs of our bodies.
- The extra food that we eat is converted into fat and stored in adipose tissues of our bodies.
- In between meals and during exercise our bodies rely on this fat to provide energy.
- In addition to that, the layer of fat under our skin protects us from cold temperatures.
- It also helps our body absorb several fat-soluble vitamins like vitamins A, D E and K.
- A layer of fat also covers our nerve cells giving them protection.
Q5. Explain the following terms: Activation energy, Transition state, Aerobic respiration
Activation Energy:
The amount of energy absorbed by reactants or products to be converted into transition state is called the activation energy ($\ce{E_a}$) of the reaction.
Activation energy is required to start a reaction and break the bonds within the reactants. For example in the formation of water, the energy required to break the bond within hydrogen and oxygen molecules is called activation energy of the reaction.
$\mathrm{H_{2(g)} + \frac{1}{2}O_{2(g)} \longrightarrow 2H_{(g)} + O_{(g)} + 684 \text{kJ}}$
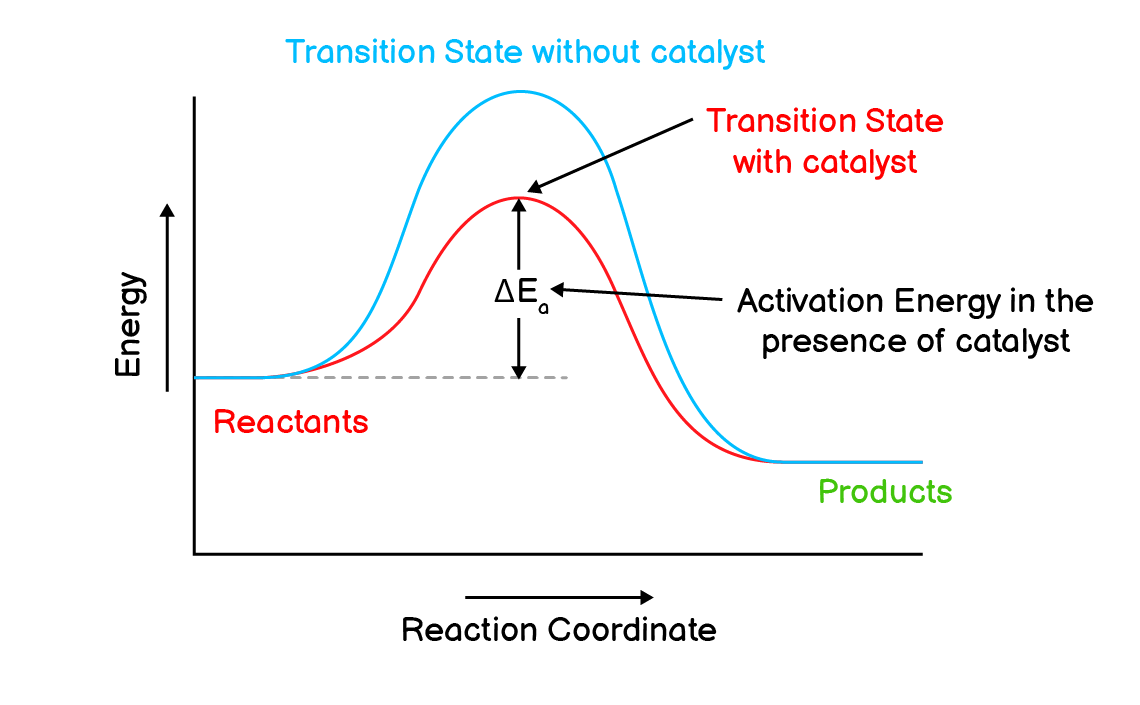
Transition State:
A high energy state that is formed due to collision between reactant molecules is called transition state.
A transition state has following properties:
- It is formed due to collision between reactant molecules with more than average kinetic energy.
- It is an intermediate between both reactants and products.
- Its energy is higher than that of both reactants and products.
- It can either return to reactants.
- Or it can convert into products.
Aerobic Respiration:
Aerobic respiration is a biochemical process that occurs in the presence of oxygen and converts glucose into carbon dioxide, water and energy.
$\mathrm{
\underset{(C_6H_{12}O_6)}{Glucose} \xrightarrow[In\ cytoplasm]{} \underset{(C_3H_4O_3)}{2\ Pyruvate} \xrightarrow[In\ mitochondria]{O_2} 6CO_{2\ (g)}\ +\ 6H_2O_{(g)}\ +\ Energy
}$
Aerobic respiration happens in the presence of oxygen and glucose is converted into carbon dioxide, water and energy.
5. Investigative Questions
Q1. Why is it essential to cook some of the food items while others we can eat without cooking?
Raw Foods:
Some food items can be eaten raw because they are easy to digest. For example, fruits and some vegetables can be eaten raw because they contain simple and easy to digest carbohydrates such glucose and fructose.
Cooked Food:
Food items are cooked to make them:
- Easier to digest
- Free from disease causing pathogens
For example, starch in potatoes and protein in meats become easier to digest when cooked at high temperatures. Without cooking our bodies would not be able to digest them properly.
Additionally, cooking these food items also kills disease causing pathogens making them safer to eat.
Q2. Why do fireworks look spectacular? What type of chemical compounds undergo chemical reactions during this activity?
Fireworks are a result of combustion reactions that produces heat, light and sound. Different metal salts and oxidizing agents produce variety of colors when burnt. For example, barium (Ba) salts produce green color, sodium (Na) salts produce yellow color and copper (Cu) salts produce blue color.