9.3.2 Ionic Bond
This is the second lecture from Chapter 3: “Chemical Bonding” in the new Class 9 Chemistry book (Punjab Board – PCTB). It explains the basics of ionic bond and formation of ionic bond in various compounds. The lecture also includes a multiple-choice quiz, short question and long question notes.
MCQs Based Quiz
Short Questions
Q1. How would you define an ionic bond, also knows as electrovalent bond?
An ionic bond is a chemical bond formed by the complete transfer of one or more electrons from one atom to another.
Example: The chemical bond in NaCl is an ionic bond.
Q2. What are ionic compounds? Give some examples of these compounds.
A compound in which atoms are held together through ionic bonds is called an ionic compound.
Example: NaCl, KCl, NaF, NaBr, CaCl2, MgF2
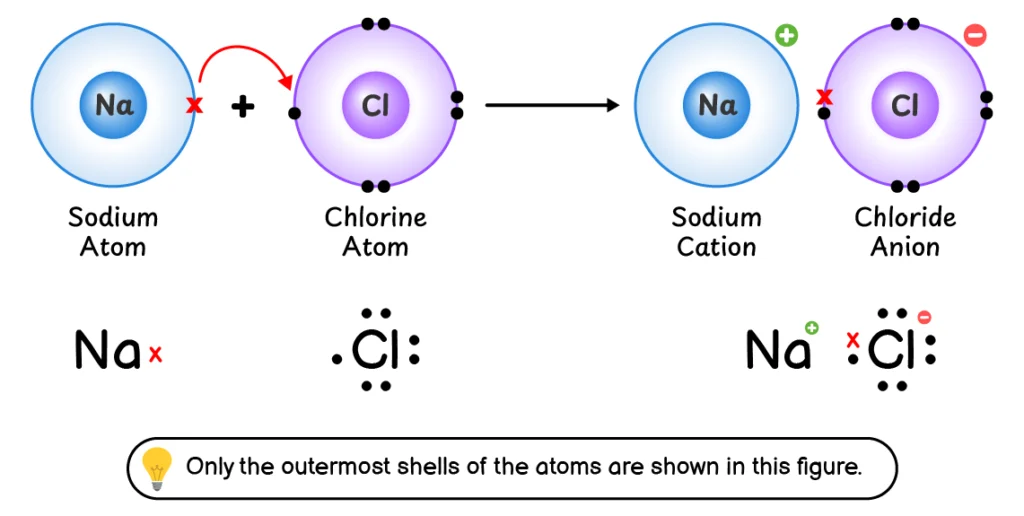
Q3. Explain the formation of chemical bond between sodium (Na) and chlorine (Cl).
The chemical bond formation involves the following steps:
- Sodium (Na) loses its outermost electron to form a Na+
- Chlorine (Cl) accepts this electron into its outermost shell to form a Cl–
- The Na+ cation and Cl– anion are held together by an electrostatic force of attraction which is called an ionic bond.
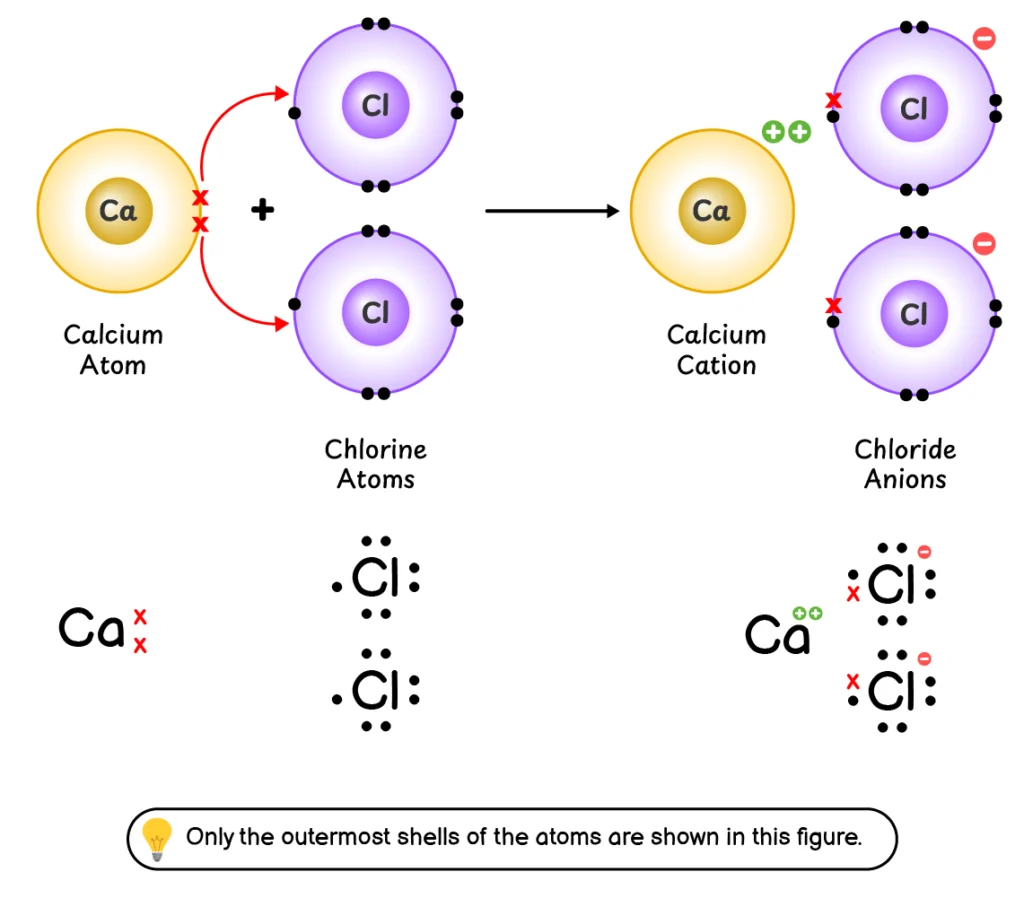
Q4. Explain the formation of chemical bond between calcium (Ca) and chlorine (Cl).
This chemical bond formation involves the following steps:
- Calcium (Ca) loses its two outermost electrons to form a Ca2+
- Two chlorine (Cl) atoms accept these two electrons into their outermost shell to form two Cl–
- Ca2+ cation and Cl– anions are held together by an electrostatic force of attraction which is called an ionic bond.
Q5. What types of elements form ionic bond?
An ionic bond is formed between the following types of atoms:
- Atoms that can complete their outermost shell by losing up to three electrons (these form cations).
- Atoms that can complete their outermost shell by gaining up to three electrons (these form anions).
Q6. What are the conditions for an ionic bond to be formed?
The following are the conditions for the formation of an ionic bond:
- The atoms involved must be able to lose or gain up to three electrons to complete their outermost shell.
- There must be a complete transfer of electrons from one atom to another.
- Electrons transference must only involve outermost shells.
Q7. What is a crystal lattice?
A crystal lattice is the three-dimensional arrangement of ions in an ionic compound.
Descriptive Question
Q1. What is an ionic or electrovalent bond? Explain the formation of ionic bond using sodium chloride (NaCl) as an example.
An ionic bond is a chemical bond formed by the complete transfer of one or more electrons from one atom to another.
Example: The chemical bond in NaCl is an ionic bond.
A compound in which atoms are held together through ionic bonds is called an ionic compound.
Example: NaCl, KCl, NaF, NaBr, CaCl2, MgF2
Elements That Form Ionic Bonds:
- Elements that can complete their outermost shell by losing up to three electrons (these form cations).
- Elements that can complete their outermost shell by gaining up to three electrons (these form anions).
Conditions for Ionic Bond Formation:
The following are the conditions for the formation of an ionic bond:
- The atoms involved must be able to lose or gain up to three electrons to complete their outermost shell.
- There must be a complete transfer of electrons from one atom to another.
- Electrons transference must only involve outermost shells.
Formation of Ionic Bond in Sodium Chloride (NaCl):
The ionic bond formation involves the following steps:
- Sodium (Na) loses its outermost electron to form a sodium cation (Na+).
$\ce{\underset{\text{(Unstable)}}{Na} -> \underset{\text{(Stable)}}{Na+} + e^-}$
- Chlorine (Cl) accepts this electron into its outermost shell to form a chloride ion (Cl–).
$\ce{\underset{\text{(Unstable)}}{Cl} + e^- -> \underset{\text{(Stable)}}{Cl-}}$
- The Na+ cation and Cl– anion are held together by an electrostatic force of attraction which is called an ionic bond.
$\ce{Na^+ + Cl^- -> NaCl }$
Q2. Explain the formation of chemical bond between calcium (Ca) and chlorine (Cl).
This ionic bond formation involves the following steps:
- Calcium (Ca) loses its two outermost electrons to form a calcium cation (Ca2+).
$\ce{
\underset{\text{(Unstable)}}{Ca} ->
\underset{\text{(Stable)}}{Ca^{2+}} + 2e^-
}$
- Two chlorine (Cl) atoms accept these two electrons into their outermost shell to form two chloride ions (Cl–).
$\ce{\underset{\text{(Unstable)}}{2Cl} + 2e^- -> \underset{\text{(Stable)}}{2Cl^-}}$
- Ca2+ cation and Cl– anions are held together by an electrostatic force of attraction which is called an ionic bond.