9.3.9 Intermolecular Forces of Attraction
This is the ninth lecture from Chapter 3: “Chemical Bonding” in the new Class 9 Chemistry book (Punjab Board – PCTB). It covers two important types of intermolecular forces: dipole-dipole forces and hydrogen bonding. The lecture also includes a multiple-choice quiz, short question and long question notes.
MCQs Based Quiz
Short Questions
Define intermolecular forces.
Intermolecular forces are the forces of attraction that exist between the molecules of elements and compounds.
Compare the intermolecular forces among the three common states of matter.
Solids have the strongest intermolecular forces, liquids have moderate intermolecular forces, and gases have the weakest intermolecular forces.
What effects do the intermolecular forces have on substances?
Intermolecular forces can influence the physical properties of substances, such as their melting and boiling points. Stronger intermolecular forces result in higher melting and boiling points.
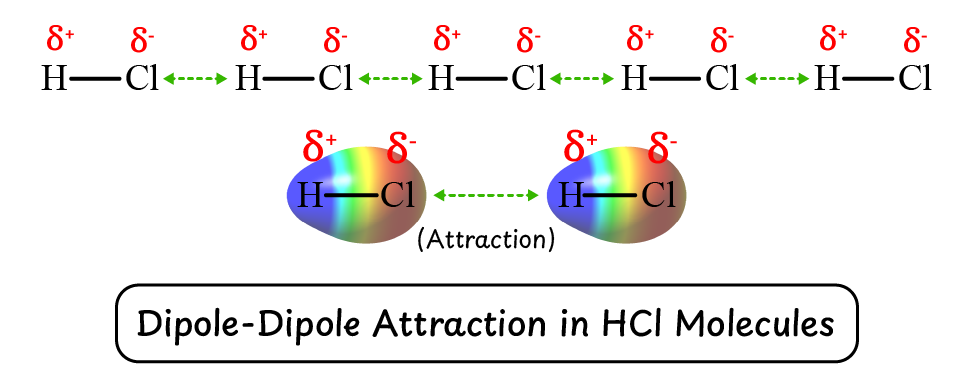
What is the definition of dipole-dipole force of attraction?
Dipole-dipole forces are attractive intermolecular forces that occur between the oppositely charged poles of polar molecules.
Example: Intermolecular force in $\ce{\overset{\delta+}{H}-\overset{\delta-}{Cl}}$.
What is required for a compound to develop dipole-dipole forces of attraction?
To develop dipole-dipole forces, a compound must be polar and possess partial charges. Example: In HCl, the shared electrons are pulled toward the more electronegative chlorine atom, creating a dipole.
How do dipole-dipole forces of attraction affect the melting and boiling points of compounds?
Dipole-dipole forces hold molecules together more strongly, which increases the melting and boiling points of the compound.
Define hydrogen bonding.
Hydrogen bonding is a special type of dipole-dipole interaction that occurs in compounds where hydrogen is covalently bonded to highly electronegative elements such as fluorine (F), oxygen (O), or nitrogen (N).
What type of intermolecular forces are present between H2O molecules?
Water (H2O) molecules exhibit hydrogen bonding because hydrogen is covalently bonded to a highly electronegative oxygen atom. This results in strong intermolecular attraction between water molecules.
What is the reason behind the high melting and boiling points of water as compared to compounds like H2S and NH3?
Water molecules form strong hydrogen bonds, which require more energy to break. This leads to higher melting and boiling points compared to H2S and NH3, which have weaker intermolecular forces.
Descriptive Question
Q1. What are intermolecular forces? Write a detailed note on dipole-dipole forces of attraction.
Intermolecular Forces:
Intermolecular forces are the forces of attraction that exist between the molecules of elements and compounds.
These forces are weaker than chemical bonds. Among the three states of matter, these forces are strongest in solids, moderate in liquids, and weakest in gases.
Intermolecular forces affect the physical properties of substances. For example, substances with strong intermolecular forces have higher melting and boiling points.
Dipole-Dipole Forces of Attraction:
Dipole-dipole forces are attractive intermolecular forces that occur between the oppositely charged poles of polar molecules.
In polar covalent compounds like $\ce{\overset{\delta+}{H}-\overset{\delta-}{Cl}}$, the shared electrons are pulled toward the more electronegative chlorine atom, creating partial charges:
- Hydrogen gets a partial positive charge ($δ^{+}$).
- Chlorine gets a partial negative charge ($δ^{-}$).
Due to these partial charges, HCl molecules attract each other, leading to the development of dipole-dipole forces.
These forces are responsible for the relatively higher melting and boiling points of polar compounds like HCl compared to non-polar compounds.
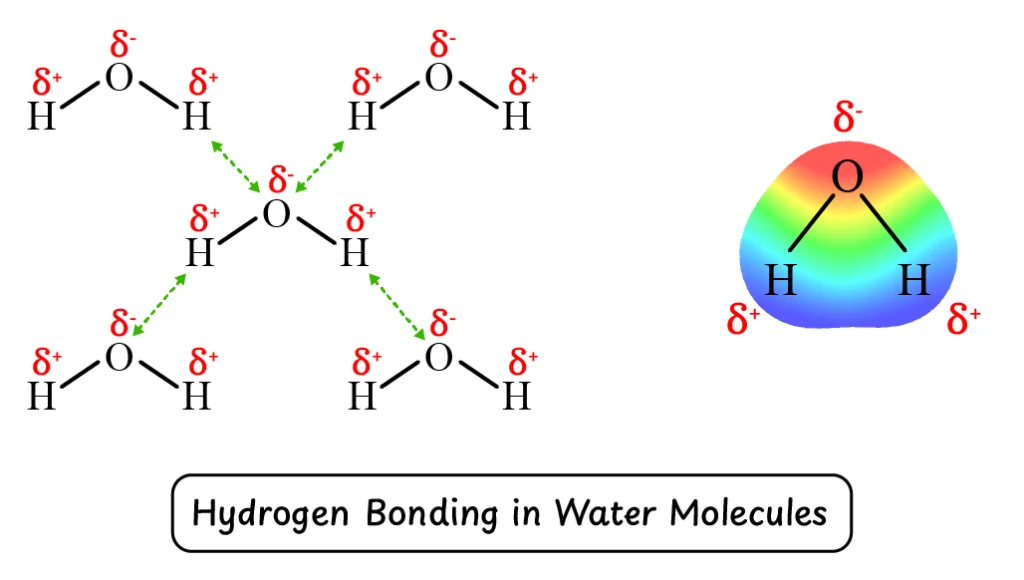
Q2. Write a comprehensive note on hydrogen bonding.
Hydrogen bonding is a special type of dipole-dipole interaction that occurs in compounds where hydrogen is covalently bonded to highly electronegative elements such as fluorine (F), oxygen (O), or nitrogen (N).
Due to the large difference in electronegativity, the covalent bond becomes highly polar, leading to the development of strong intermolecular attractions known as hydrogen bonds.
For example, in H2O, the O-H bonds are highly polar, which is the cause of hydrogen bonding between water molecules. This hydrogen bonding is the reason why water has relatively higher melting and boiling points compared to similar compounds like H2S and NH3.