9.3.3 Covalent Bond
This is the third lecture from Chapter 3: “Chemical Bonding” in the new Class 9 Chemistry book (Punjab Board – PCTB). It covers the basics of covalent bond and formation of covalent bond in various compounds. The lecture also includes a multiple-choice quiz, short question and long question notes.
MCQs Based Quiz
Short Questions
Q1. Define Covalent Bond.
A covalent bond is a chemical bond in which two atoms share an electron pair between them.
Example: All chemical bonds in H2O are covalent bonds.
Q2. What is a bond pair?
The pair of electrons that is shared by both atoms is called bond pair.
Q3. Define single covalent bond.
A single covalent bond is a bond in which both atoms share a single electron pair. It is represented by a single line (—).
Example: H—H.
Q4. Define double covalent bond.
A double covalent bond is a bond in which both atoms share two electron pairs. It is represented by double lines (═)
Example: O═O
Q5. Define triple covalent bond.
A triple covalent bond is a bond in which both atoms share three electron pairs. It is represented by triple lines (≡).
Example: N≡N
Q6. How does covalent bond lower the energy of atoms?
During covalent bond formation, the bond pair electrons are attracted by the nuclei of both atoms. This increased attraction lowers the energy of both atoms, making them more stable.
Q7. What causes the attractive and repulsive forces between atoms during covalent bond formation?
- Attractive forces are developed when the electrons of one atom are attracted by the nucleus of another atom.
- Repulsive forces are developed due to the repulsion between the electrons and nuclei of both atoms.
Q8. How do atoms lower the repulsive forces to develop a covalent bond between them?
Atoms adjust to an optimum distance where attractive forces dominate over repulsive forces. At this distance, a stable covalent bond is formed.
Q9. What type of elements forms covalent bonds?
Covalent bonds are formed between elements that cannot afford complete electron transfer. These atoms achieve stability by sharing up to three bond pairs of electrons.
Q10. How is a covalent bond different from an ionic bond?
- In an ionic bond, there is a complete transfer of electrons from one atom to another.
- In a covalent bond, atoms mutually share one, two, or three pairs of electrons.
Q11. How is a water molecule formed?
A water molecule (H2O) is formed when the oxygen atom shares one bond pair with each of the two hydrogen atoms.
Q12. How is a carbon dioxide molecule formed?
A carbon dioxide (CO2) molecule is formed when each of the two oxygen atoms shares two bond pairs of electrons with the central carbon atom.
Q13. How is a methane molecule formed?
A methane molecule (CH4) is formed when one carbon atom shares four bond pairs of electrons with four hydrogen atoms.
Q14. Draw electron dot and cross structures of the following compounds.
SiH4, PCl3, SO2, SO3, NH3 and HCN
Descriptive Question
Q1. Write a detailed note on covalent bond.
A covalent bond is a chemical bond in which two atoms share an electron pair between them.
The pair of electrons that is shared by both atoms is called bond pair.
Formation of Covalent Bond:
- Two atoms approach each other.
- The electrons of one atom are attracted by the nucleus of the other atom.
- The electrons and nucleus of one atom repel the electrons and nucleus of the other atom.
- The atoms adjust to an optimum distance where attractive forces dominate repulsive forces.
- At this distance, both atoms have minimum energy.
- Due to the shared pair of electrons, both atoms achieve the electronic configuration of the nearest noble gas, which gives them stability.
- This leads to the formation of a covalent bond.
Types of Covalent Bonds:
There are three types of covalent bonds:
- Single covalent bond (—): Both atoms share one bond pair.
Example: H—H - Double covalent bond (═): Both atoms share two bond pairs.
Example: O═O - Triple covalent bond (≡): both atoms share three bond pairs.
Example: N≡N
Q2. Explain the formation of a single, double and covalent bond using dot-and-cross structures.
Single Covalent Bond:
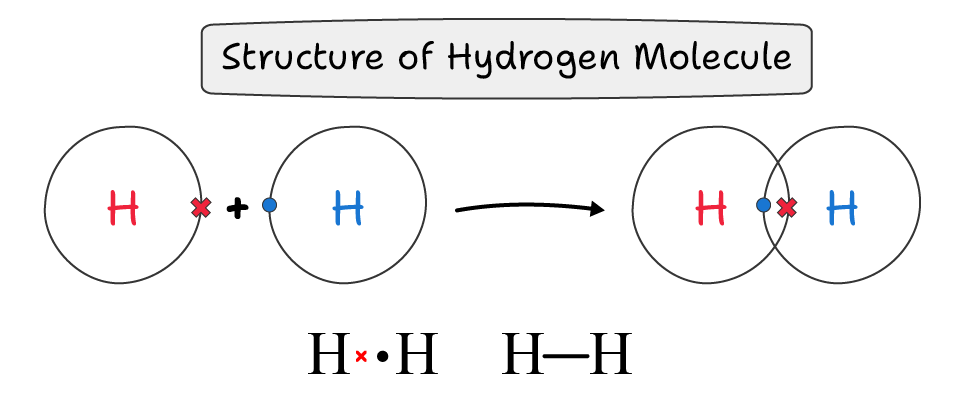
A single covalent bond is a bond in which both atoms share a single electron pair. It is represented by a single line (—).
For example, in a hydrogen molecule (H—H), both atoms share one bond to form a single covalent bond.
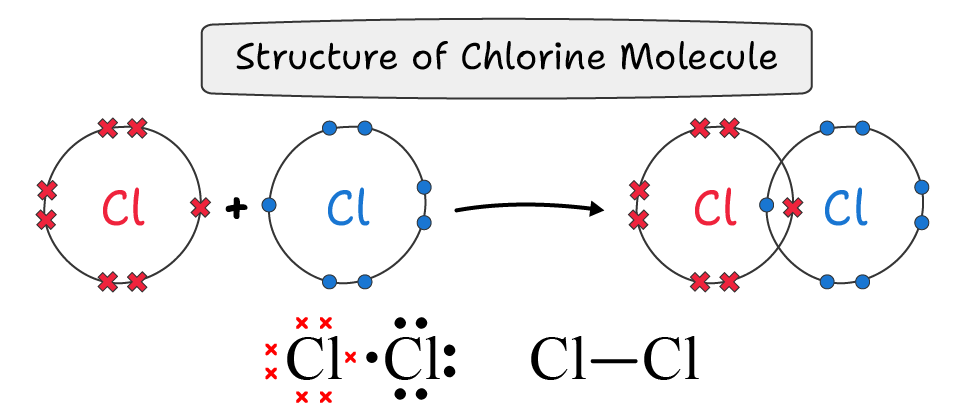
Similarly, in two chlorine atoms also share one bond pair to form a single covalent bond between them.
Similarly, in two chlorine atoms also share one bond pair to form a single covalent bond between them.
Double Covalent Bond:
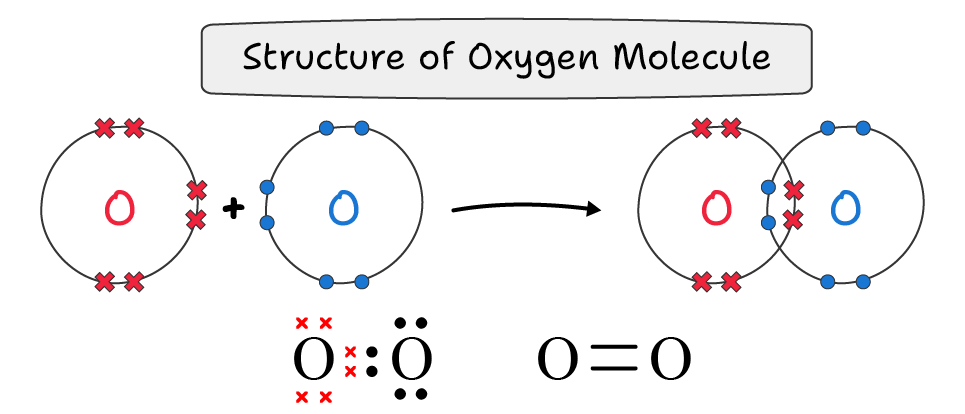
A double covalent bond is a bond in which both atoms share two electron pairs. It is represented by double lines (═)
For example, an oxygen molecule (O═O) is formed by the sharing of two bond pair electrons between oxygen atoms.
Triple Covalent Bond:
A triple covalent bond is a bond in which both atoms share three electron pairs. It is represented by triple lines (≡).
For example, in nitrogen molecule (N≡N), two nitrogen atoms are bonded together by a triple covalent bond.

Q2. How would you explain the formation of covalent bonds in water, carbon dioxide, methane, ethene and methanol.
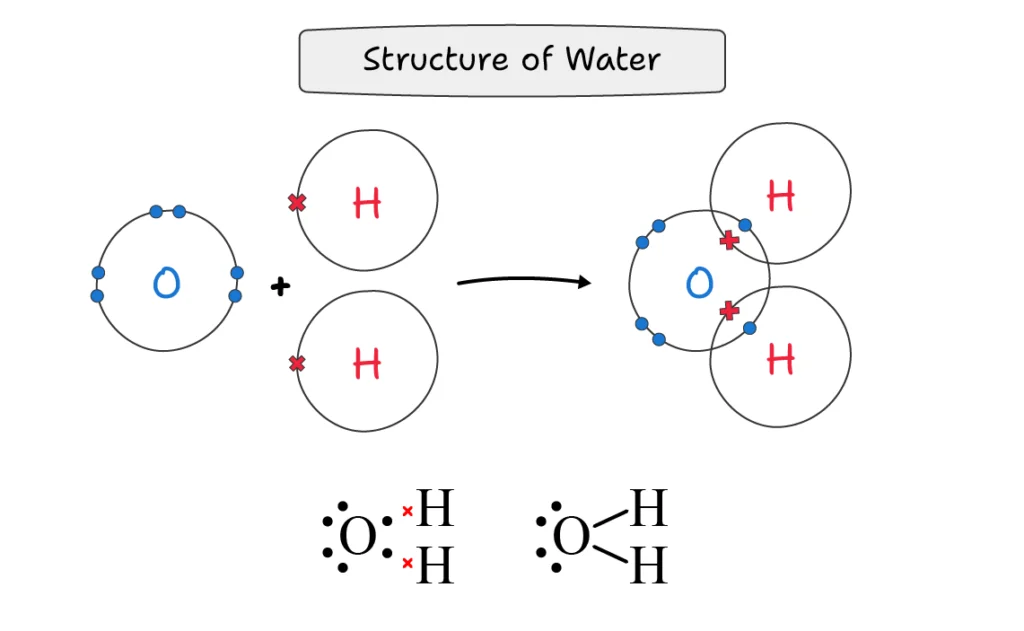
Water (H2O):
A water molecule is formed when the oxygen atom shares one bond pair with each of the two hydrogen atoms.
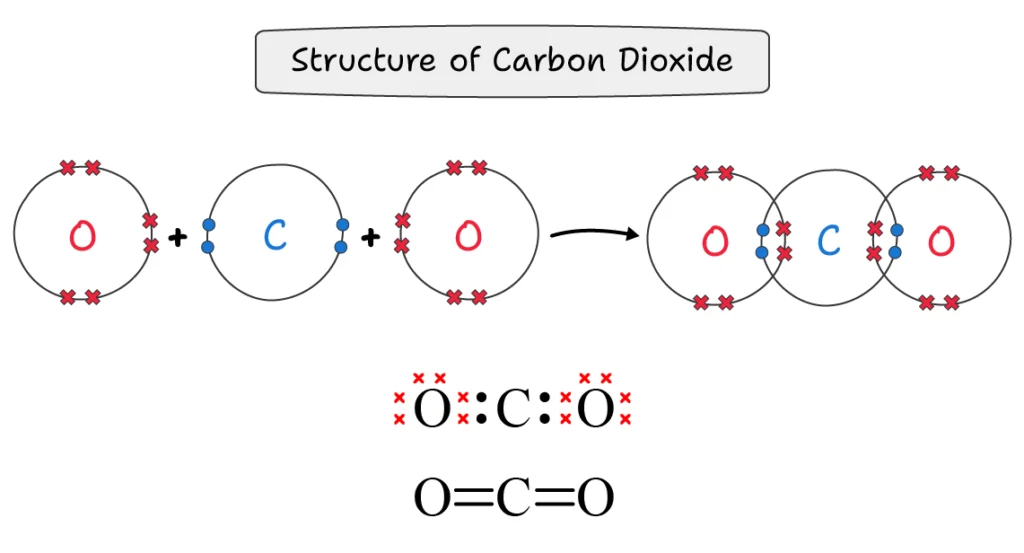
Carbon Dioxide (CO2):
A carbon dioxide molecule is formed when each of the two oxygen atoms shares two bond pairs with the central carbon atom. As a result, the carbon atom forms two double bonds with the adjacent oxygen atoms.
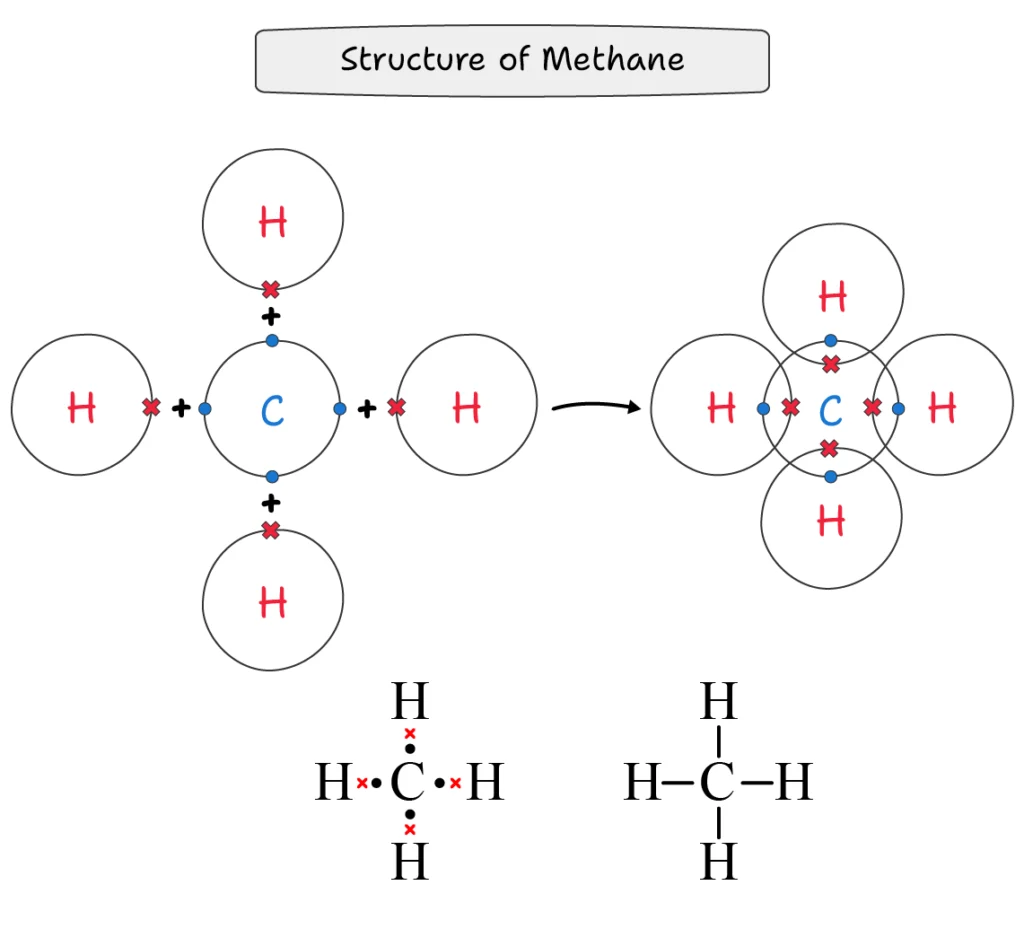
Methane (CH4):
A methane molecule is formed when one carbon atom shares four bond pairs with four hydrogen atoms.
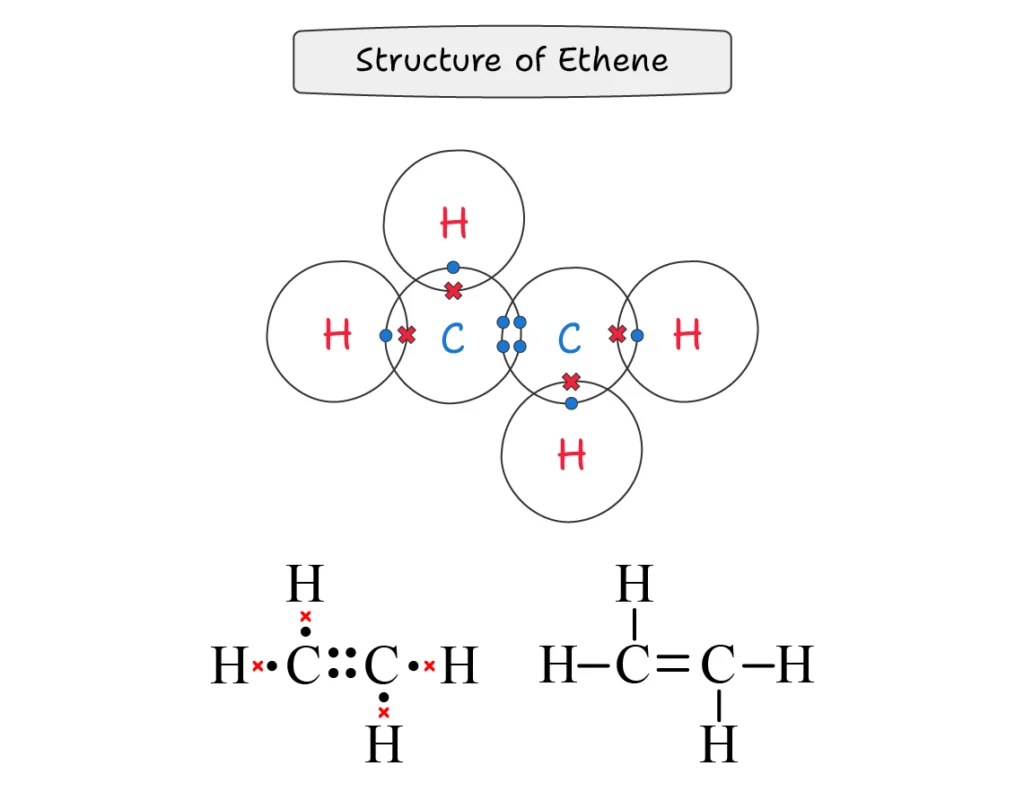
Ethene (C2H4):
In ethene molecule, two carbon atoms make a double bond between them, and two hydrogen atoms are also attached with each of these two carbon atoms via a single covalent bond.
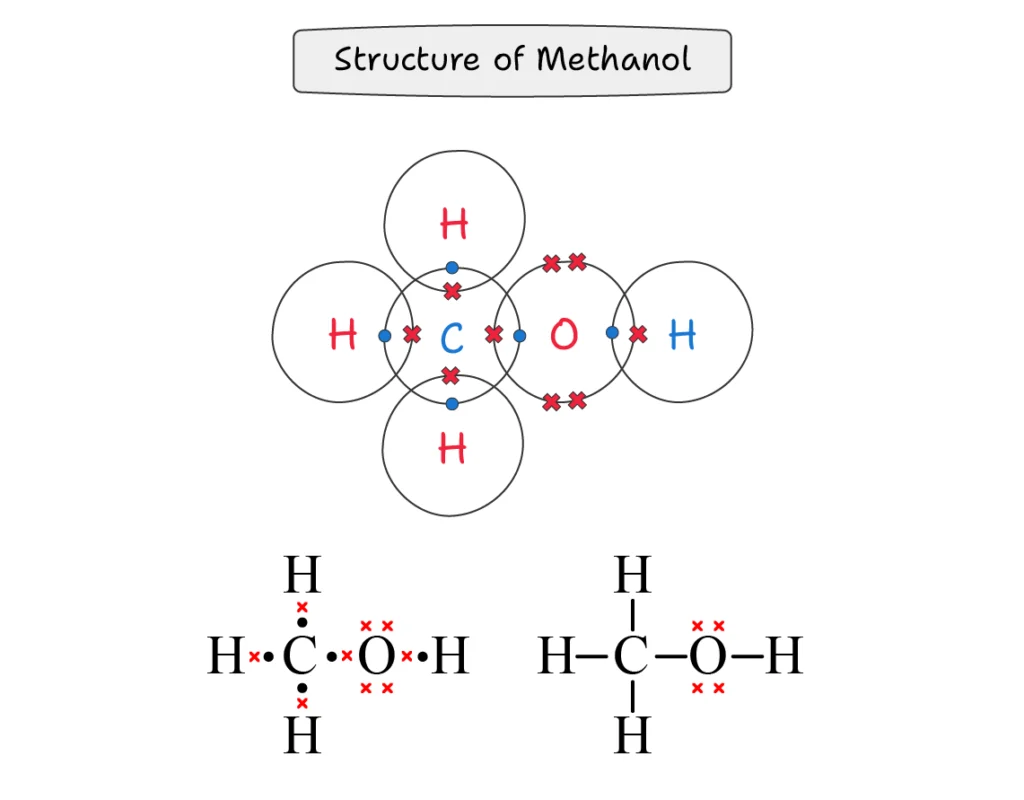
Methanol (CH3OH):
In methanol molecule, carbon atom is attached to three hydrogen atoms through a covalent bond, and it also makes a single covalent bond with oxygen. The oxygen atom makes another single covalent bond with a hydrogen atom.