Chapter 3: Chemical Bonding (Solved Exercise Notes)
This is solved exercise notes for chapter 3: ‘Chemical Bonding’ of the new book for Class 9 Chemistry Punjab Board (PCTB) 2025. These notes include solved multiple choice questions (MCQs), questions for short answers, constructed response questions, descriptive questions and investigative questions.
1. Multiple Choice Questions
1. When molten copper and molten zinc are mixed together, they give rise to a new substance called brass. Predict what type of bond is formed between copper and zinc.
(a) Coordinate covalent bond
(b) Ionic bond
(c) Metallic bond
(d) Covalent bond
2. Which element is capable of forming all the three types of bonds; covalent, coordinate covalent and ionic?
(a) Carbon
(b) Oxygen
(c) Magnesium
(d) Silicon
3. Why is H2O a liquid while H2S is a gas?
(a) Because in water, the atomic size of oxygen is smaller than that of sulphur
(b) Because water is a polar compound and there exists strong forces of attraction between its molecules
(c) Because H2O molecule is lighter than H2S
(d) Because water can easily freeze into ice
4. Which of the following bonds is expected to be the weakest?
(a) C-C
(b) Cl-Cl
(c) O-O
(d) F-F
5. Which form of carbon is used as a lubricant?
(a) Coal
(b) Diamond
(c) Graphite
(d) Charcoal
6. Keeping in view the intermolecular forces of attraction, indicate which compound has the highest boiling point.
(a) H2O
(b) H2S
(c) HF
(d) NH3
7. Which metal has the lowest melting point?
(a) Li
(b) Na
(c) K
(d) Rb
8. Which ionic compound has the highest melting point?
(a) NaCl
(b) KCl
(c) LiCl
(d) RbCl
9. Which compound contains both covalent and ionic bonds?
(a) MgCl2
(b) NH4Cl
(c) CaO
(d) PCl3
10. Which among the following has a double covalent bond?
(a) Ethane
(b) Methane
(c) Ethylene
(d) Acetylene
2. Questions for Short Answers
Q1. What type of elements loses their outer electron easily and what type of elements gain electron easily?
Metals such as, Na, K and Fe, can easily lose their outermost electrons while non-metals, such as F, Cl and Br, can easily gain electrons.
Q2. Why do lower molecular mass covalent compounds exist as gases or low boiling liquids?
Most lower molecular mass covalent compounds do not have strong intermolecular forces that can bind their molecules together. Therefore, such compounds exist as low boiling temperature gases.
Q3. Give one example of an element which exists as a crystalline solid and it has covalent bonds between its atoms.
Carbon element has crystalline forms such as graphite and diamonds in which atoms are covalently bonded.
Q4. Which property of metals makes them malleable and ductile?
When pressure is applied on the metals, their upper rows of atoms slip pass the lower rows. This property make them malleable and ductile.
Q5. Is coordinate covalent bond a strong bond?
After the formation of a coordinate covalent bond, there is no difference between a coordinate covalent and a common covalent bond. So they both have similar strength. But compared to ionic bond, coordinate covalent bond is weaker.
Q6. Write down dot and cross formula of HNO3.
3. Constructed Response Questions
Q1. Why HF is a liquid while HCl is a gas?
HF is a liquid because it has hydrogen bonding between its molecules as compared to dipole-dipole forces between HCl molecules which is weaker than hydrogen bonding.
Q2. Why covalent compounds are generally not soluble in water?
Most covalent compounds have a non-polar bond with no partial charges. Due to absence of these partial charges, polar molecules such as water cannot dissolve them.
Q3. How do metals conduct heat?
Metals conduct heat due to their sea of free electrons. These free electrons conduct heat from one part of the metal to the other part.
Q4. How many oxides does nitrogen form? Write down the formulae of oxides?
Nitrogen forms oxides with the following formulae:
N2O, NO, N2O3, NO2, N2O4, and N2O5
Q5. What will happen if NaBr is treated with AgNO3 in water?
This will lead to an exchange reaction where both of these ionic compounds exchange their positive and negative ions.
$\ce{
NaBr_{(aq)} + AgNO_{3 (aq)} -> AgBr_{(s)} + NaNO_{3 (aq)}
}$
Q6. Why does iodine exist as a solid while Cl2 exist as a gas?
Due to larger size of iodine, there exist a stronger intermolecular force between I2 molecules. This stronger intermolecular force causes iodine to exist as solid, compared to Cl2 which exist as a gas.
4. Descriptive Questions
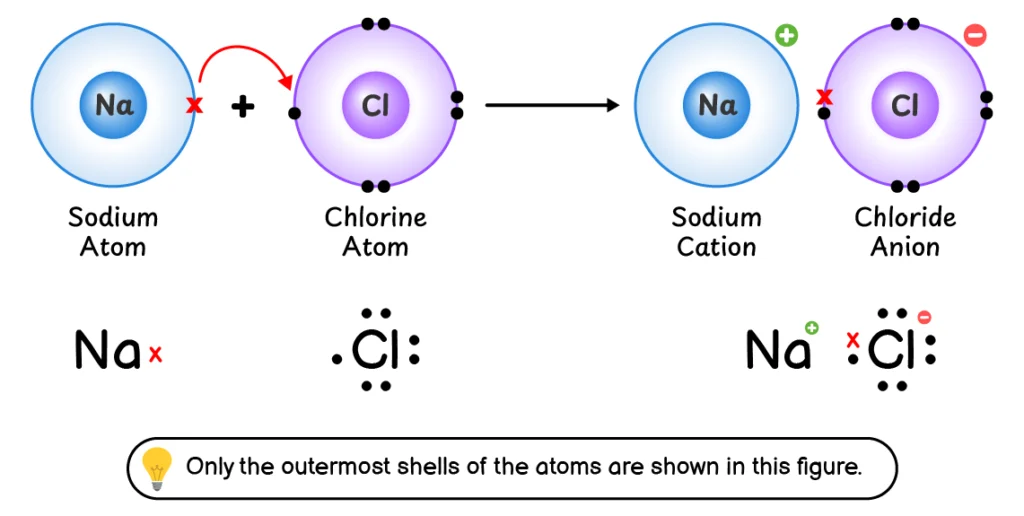
Q1. Explain the formation of an ionic bond and a covalent bond.
Formation of Ionic Bond:
An ionic bond is a chemical bond formed by the complete transfer of one or more electrons from one atom to another.
The ionic bond formation involves the following steps:
- Sodium (Na) loses its outermost electron to form a Na+ cation.
$\ce{\underset{\text{(Unstable)}}{Na} -> \underset{\text{(Stable)}}{Na+} + e^-}$
- Chlorine (Cl) accepts this electron into its outermost shell to form a Cl–
$\ce{\underset{\text{(Unstable)}}{Cl} + e^- -> \underset{\text{(Stable)}}{Cl-}}$
- The Na+ cation and Cl– anion are held together by an electrostatic force of attraction which is called an ionic bond.
$\ce{Na^+ + Cl^- -> NaCl }$
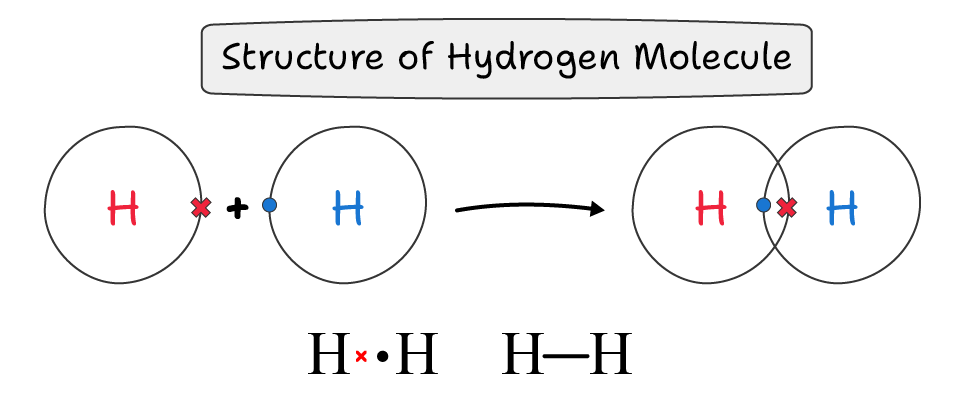
Formation of Covalent Bond:
A covalent bond is a chemical bond in which two atoms share an electron pair between them.
The pair of electrons that is shared by both atoms is called bond pair.
The covalent bond formation involves the following steps:
- Two hydrogen atoms approach each other.
- The electrons of one atom are attracted by the nucleus of the other atom.
- The electrons and nucleus of one atom repel the electrons and nucleus of the other atom.
- The atoms adjust to an optimum distance where attractive forces dominate repulsive forces.
- At this distance, both atoms have minimum energy.
- Due to the shared pair of electrons, both atoms achieve the electronic configuration of the nobel gas helium, which gives them stability.
- This leads to the formation of a covalent bond.
Q2. How do ions arrange themselves to form NaCl crystal.
In NaCl, oppositely charged ions (Na+ and Cl–) are held together by strong electrostatic forces of attraction. These forces create a crystal lattice in which the opposite ions are arranged in a regular, repeating three-dimensional pattern. Because these opposite ions are spherical in shape, they can surround each other from all directions, making ionic bond non-directional.
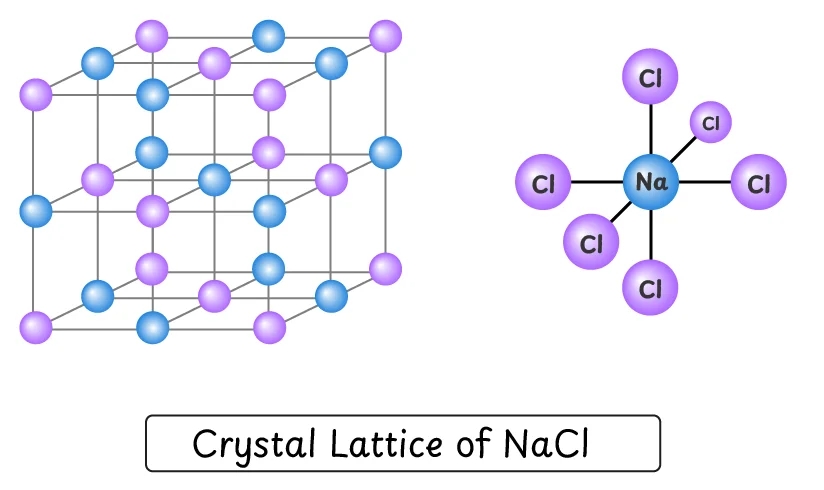
In a crystal lattice of NaCl, each Na+ ion is surrounded by chloride (Cl–) from all 6 directions: top, bottom, front, back, left and right. Similarly, each chloride (Cl–) ion is also surrounded by Na+ from all 6 sides.
This alternate arrangement of equal number of oppositely charged ions makes the whole crystal lattice electrically neutral. This crystal lattice is however extremely brittle, and an external force can easily break it.
Q3. Explain the properties of metals keeping in view the nature of metallic bond.
Hardness:
In metals, atoms are firmly held in place due to strong metallic bonding. These tightly held atoms form stacked layers. Because of this strong bonding, metals can withstand very large forces without breaking.
Thermal and electrical conduction:
In metals, the outer shell electrons leave their atom and move freely in the empty spaces between the metal atoms. Due to these free electrons, metals are very good conductors of heat and electricity.
Malleability and Ductility:
Metals also consist of layers of closely packed atoms held together by strong metallic bonds. When pressure is applied, these layers slide over each other. This ability gives metals their properties of malleability and ductility.
High melting and boiling point:
Melting and boiling involve the breaking of chemical bonds between atoms, molecules or ions. But because metals have very strong metallic bonding, they have very high melting and boiling points.
Heaviness:
The strong metallic bonding also causes metal atoms to be tightly packed together. As a result, more atoms are packed into a small volume, which increases the metal’s density. This high density is the reason why metals often feel heavy.
Q4. Compare the properties of ionic and covalent compounds.
Ionic Compounds
Covalent Compounds
In ionic compounds, oppositely charged ions are perfectly arranged in a crystalline structure. As a whole, the compound is neutral.
Covalent compounds mostly exist as individual neutral units.
They are made of at least one metal and one non-metal.
They are made of two or more non-metals.
Electrostatic forces of attraction exist between the cations and anions.
Electrostatic force of attraction exists between nuclei and shared electrons.
They usually have very high melting and boiling points due to strong electrostatic attractions between ions, which are difficult to break. For example, the melting point of NaCl is 801°C.
Their lower molecular mass compounds are gases and have low melting and boiling points. High molecular mass compounds are solids, but they also have lower melting and boiling points than ionic compounds.
Most ionic compounds are solids at room temperature. Example: sodium chloride (NaCl).
They exist in all states, i.e., solid, liquid and gas.
They are generally soluble in polar solvents, such as water.
Polar compounds are soluble in polar solvents, while non-polar compounds are soluble in non-polar solvents.
They are good conductors of electricity in molten or aqueous solution form, because in these states, the ions are free to move.
They are usually bad conductors of electricity.
Examples: NaCl, KCl, CaCl2
Examples: H2O, CO2 and HCl
Q5. How will you explain the electrical conductivity of graphite crystals?
Electrical Conductor:
When a conductor is connected to an electric source, the electrons or ions are pushed from one part of the conductor to another part. This flow of electrons or ions is called electricity. Any material that allows electrons or ions to flow through it is called an electrical conductor.
Electrical Conductivity of Graphite:
In graphite, carbon atoms form stacked layers in which each atom is bonded to three other carbon atoms in a hexagonal pattern. Carbon has a valency of four, meaning it has four electrons in its outermost shell. However, in this bonding pattern, only three of the four electrons are used in covalent bonds.
The remaining one electron per carbon atom does not participate in bonding and becomes a free electron (similar to metals). When a piece of graphite is connected to an electric source, these free electrons are pushed from one end to another, resulting in the flow of electricity. This is why graphite, despite being a covalent substance, is considered a good electrical conductor.
Q6. Why are metals usually hard and heavy?
In metals, atoms are firmly held in place due to strong metallic bonding. These tightly held atoms form stacked layers. Because of this strong bonding, metals can withstand very large forces without breaking. When pressure is applied, instead of breaking like brittle ionic compounds, the layers of metal atoms slide over each other. This movement allows metals to disperse the pressure and gives them flexibility and strength.
The strong metallic bonding also causes metal atoms to be tightly packed together. As a result, more atoms are packed into a small volume, which increases the metal’s density. This high density is the reason why metals often feel heavy.
5. Investigative Questions
Q1 The formula of AlCl3 in vapour phase is Al2Cl6 which means it exists as dimer. Explain the bonding between its two molecules.
Bonding in AlCl3 molecule:
In AlCl3 molecules, all three chlorine atoms share one electron with aluminium atom to complete their octet. However, the aluminium atom still has an incomplete octet with 6 electrons in its outermost shell.
Bonding in Al2Cl6:
In Al2Cl6, two AlCl3 molecules combine through coordinate covalent bond to complete the octet of central aluminium atoms. The chlorine atom of one AlCl3 molecule donates the bond pair to the other AlCl3 molecule and vice versa.
Q2. Explain the structure of sand (SiO2).
Silicon has four electrons in its outermost shell. Whereas oxygen has 6 electrons in its outermost shell. In a single molecule of SiO2, two oxygen atoms are bonded via double bonds with central silicon atom.
However, in sand, multiple SiO2 units are interconnected with each other making a large covalent structure. In this large covalent structure, one silicon atom is bonded with four oxygen atoms. This forms a large three-dimensional network of covalent bonds.