9.2.3 Discovery of Protons
This is the third lecture from Chapter 2: ‘Atomic Structure’ of the new book for Class 9 Chemistry (Punjab Board – PCTB). It discusses the discovery of protons. The lecture includes a multiple-choice quiz, short-answer questions, and detailed long-answer notes.
MCQs Based Quiz
Short Questions
Q1. What is the reason behind electrical neutrality of atoms?
Atoms are neutral because they contain an equal number of positively charged protons and negatively charged electrons.
Q2. Describe the construction of the modified discharge tube used by E. Goldstein in his experiments to study canal rays.
- Goldstein used a discharge tube that contained a perforated cathode.
- When a high voltage was applied, canal rays were produced and passed through the holes in the cathode.
Q3. Describe the properties of canal rays.
- Canal rays are positively charged particles that move towards cathode.
- They are gas cations, and their properties depend on the type of gas used in the discharge tube.
Q4. What was the contribution of Ernest Rutherford in the discovery of proton?
In 1917, Ernest Rutherford proved that hydrogen nucleus (proton) is present in the nuclei of all other elements. He proposed that this hydrogen nucleus is a building block of all the nuclei.
Descriptive Question
Q1. Write a note on the discovery of protons.
The discovery of protons is mainly attributed to E. Goldstein’s discharge tube experiment and Ernest Rutherford’s gold foil experiment.
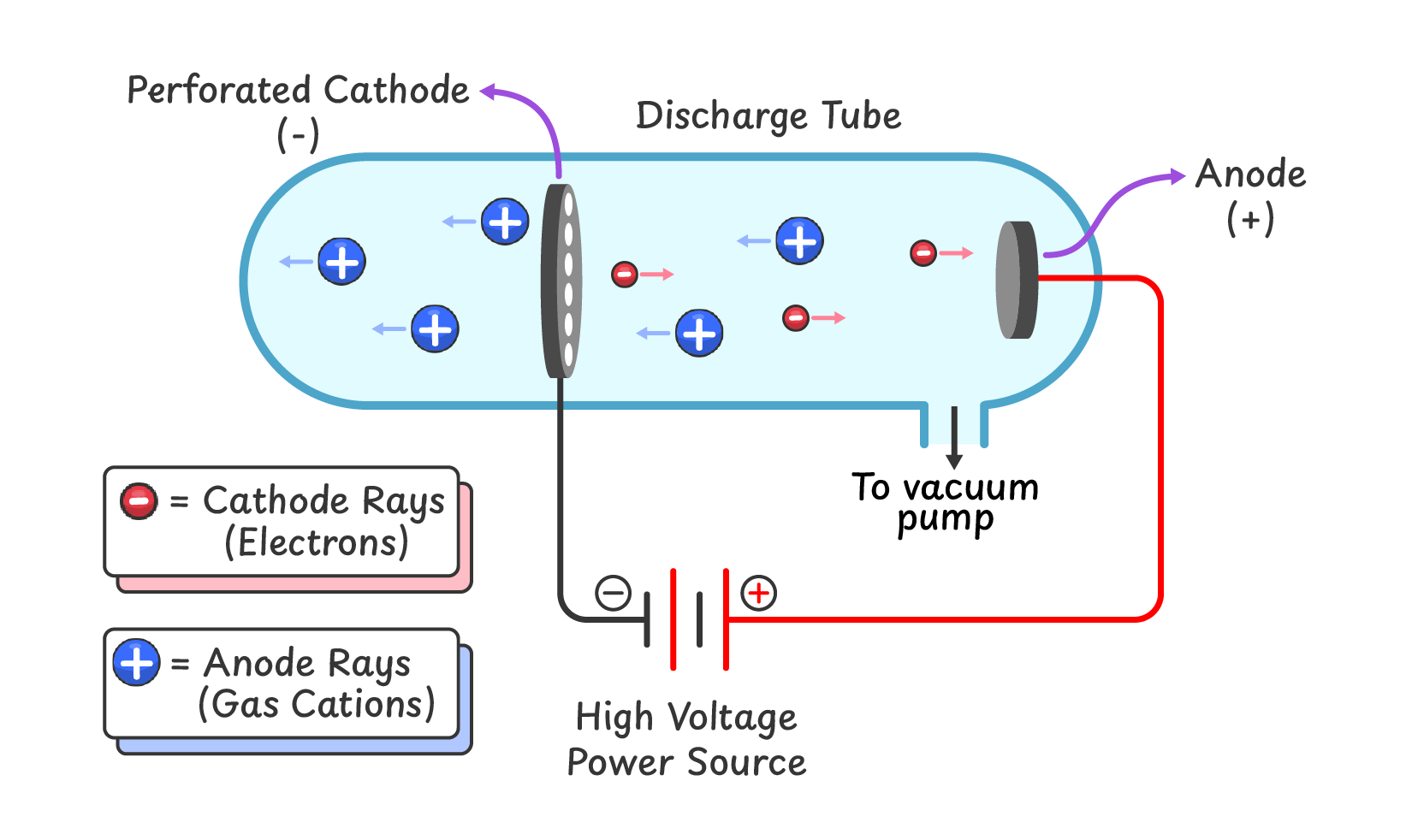
Goldstein’s Observations:
- Goldstein used a modified discharge tube equipped with a perforated cathode. In these experiments he observed the following:
- A new type of rays was produced that traveled from the anode to the cathode through the perforations. These were called anode rays or canal rays.
- The properties of these gases depended on the type of gas used in the discharge tube.
- These rays were deflected towards the negatively charged plate, indicating that they were made of positively charged particles (gas cations).
Rutherford’s Observations:
In 1917, Ernest Rutherford conducted experiments that led to the identification of the proton as a fundamental part of atomic structure. He observed that:
- Hydrogen nuclei were present in all types of nuclei.
- He concluded that this hydrogen nucleus is a basic building block of all atomic nuclei, and named it the proton.