Chapter 2: Atomic Structure (Solved Exercise Notes)
This is solved exercise notes for chapter 2: ‘Atomic Structure’ of the new book for Class 9 Chemistry Punjab Board (PCTB) 2025. These notes include solved multiple choice questions (MCQs), questions for short answers, constructed response questions, descriptive questions and investigative questions.
1. Multiple Choice Questions
1. How many electrons can be accommodated at the most in the third shell of the elements?
(a) 8
(b) 18
(c) 10
(d) 32
2. What information was obtained from discharge tube experiments?
(a) Structure of atom was discovered
(b) Neutrons and protons were discovered
(c) Electrons and protons were discovered
(d) Presence of nucleus in an atom was discovered.
3. Why have isotopes not been shown in the periodic table?
(a) Periodic table cannot accommodate a large number of isotopes of different elements.
(b) Some of the isotopes are unstable and they give rise to different elements
(c) All the isotopes have same atomic number; so there is no need to give them separate places.
(d) Isotopes do not show periodic behavior.
4. Which particle is present in different number in the isotopes?
(a) Electron
(b) Neutron
(c) Proton
(d) Both neutron and electron
5. In which isotope of oxygen are there equal numbers of protons, electrons, and neutrons?
(a) 17O
(b) 16O
(c) 18O
(d) None of these
6. What will be the relative atomic mass of nitrogen given the abundances of its two isotopes, 14N and 15N, are 99.64% and 0.35% respectively?
(a) 14.0210
(b) 14.0021
(c) 14.2100
(d) 14.1200
7. How is radiocarbon dating useful for archaeologists?
(a) It helps determine the age of organic matter.
(b) It helps determine the composition of matter.
(c) It helps determine the usefulness of matter.
(d) It helps determine whether the matter is radioactive or not.
8. What keeps the particles present in the nucleus intact?
(a) Particles are held together by strong nuclear force.
(b) Particles are held together by weak nuclear force.
(c) Particles are held together by electrostatic force.
(d) Particles are held together by dipolar force.
9. How do electrons keep themselves away from the oppositely charged nucleus?
(a) By keeping themselves stationary
(b) By revolving around the nucleus
(c) Due to their wave-like nature
(d) A magnetic field around the nucleus keeps them away
10. Rubidium consists of two isotopes, 85Rb and 87Rb. The percent abundance of light isotope is 72.2%. What is the percent abundance of the heavier isotope? Its atomic mass is 85.47.
(a) 15%
(b) 27.8%
(c) 37%
(d) 72%
2. Questions for Short Answers
Q1. Why is it said that almost all the mass of an atom is concentrated in its nucleus?
Almost 99.9% mass of an atom comes from protons and neutrons, which are located in the nucleus. Therefore, it is said that nearly all the mass of an atom is concentrated in its nucleus.
Q2. Why are elements different from one another?
Elements differ from each other due to their different number of protons, known as the atomic number. This unique number gives each element its specific properties.
Q3. How many neutrons are present in ${\Large ^{210}_{83}Bi}$ ?
To calculate the number of neutrons in a bismuth atom, subtract its atomic number (Z) from its mass number (A):
$
\begin{aligned}
\text{Neutron number (N)} &= A \;- \;Z \\
&= 210 \; – \; 83 \\
&= \boxed{127}
\end{aligned}
$
So, this bismuth atom contains 127 neutrons.
Q4. Why is tritium (${\Large ^{3}_{1}H}$) a radioactive element?
Tritium has an unstable nucleus because it contains two neutrons and only one proton. This imbalance makes the nucleus unstable, so tritium is a radioactive isotope.
Q5. How can an atom absorb and release energy?
Atoms can release or absorb energy either through their electrons or through their nucleus.
- When electrons change energy levels (shells), they either absorb or release energy.
- The nucleus can also absorb or release energy, such as during radioactive decay.
3. Constructed Response Questions
Q1. Why does the energy of electron increase as we move from the first shell to the second shell?
The energy of an electron depends on its distance from the nucleus. As an electron moves farther away, its energy increases. Therefore, when an electron moves from the first shell (closer to the nucleus) to the second shell (farther from the nucleus), its energy increases because of the greater distance.
Q2. Why is it needed to lower the pressure of the gas inside the discharge tube?
In a cathode ray tube, electrons move from the cathode to the anode. To make their journey easier, the gas pressure is kept low so that fewer gas molecules collide with the passing electrons.
Q3. What is the classical concept of an electron? How has this concept changed with time,
The classical concept of electron considers it as just a particle. With the development of science, scientists came to know that an electron actually behaves as both — as a particle as well as a wave. Its exact location cannot be determined with 100% accuracy.
Q4. Why the nuclei of the radioactive elements are unstable?
The nuclei of radioactive isotopes are unstable because of an imbalance between the number of neutrons and protons. Nuclei require a specific ratio of neutrons and protons. If a nucleus does not have this ratio, it can become unstable.
Q5. During the discharge tube experiments, how did the scientists conclude that same type of electrons and protons are present in all the elements?
In discharge tube experiments, scientists saw that cathode and anode rays behaved the same with all materials. Cathode rays went to the positive plate, anode rays to the negative. Therefore, they concluded that all elements contain the same kind of electrons and protons.
4. Descriptive Questions
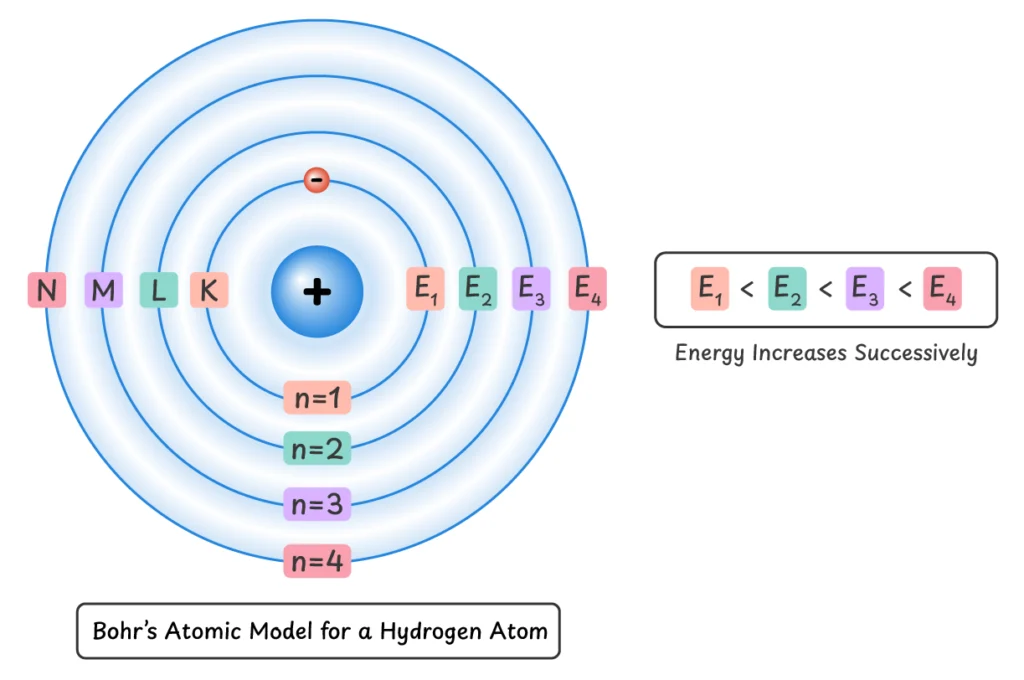
Q1. Explain the structure of hydrogen atoms.
In 1913, Niels Bohr proposed an atomic model for hydrogen atom which is called Bohr’s atomic model. The model has the following postulates:
- Electrons can only revolve around the nucleus in specific paths called shells, orbits or energy levels.
- They cannot occupy any space in between these orbits.
- An electron revolving in one of these orbits has fixed energy.
- These shells or energy levels can be represented by letters (K, L, M, N, …) or numbers (1, 2, 3, 4, …).
- The orbit closest to the nucleus has the minimum energy.
- As the distance from the nucleus increases, the energy of the orbits also increases.
- The state of an atom when its electrons are closest to the nucleus is called the ground state.
- Because electrons in these orbits have fixed energy, they are also called energy levels.
Q2. How does the theory of atomic structure explain the ionization of atoms by a radioactive isotope?
Ionization is the process of removing one or more outermost electrons from an atom. This removal requires a certain amount of energy known as ionization energy.
Atomic Structure:
- Nucleus of an atom exerts an attractive force on the surrounding electrons.
- Electrons move around the nucleus in specific energy levels or shells.
- Electrons in shells closer to the nucleus experience a stronger attraction than those in outer shells.
- For an electron to jump from a lower energy level to a one, it must absorb energy from its surroundings.
Radioactive Isotopes and Ionization:
Isotopes of an element that have an unstable nucleus and can release energy in the form of radiation are called radioactive isotopes.
When an atom is exposed to this radiation from such isotopes:
- Its electrons absorb energy from the radiation.
- This causes electrons to jump from lower to higher energy levels.
- However, if the absorbed energy is equal to or greater than the atom’s ionization energy, the electron can completely escape the atom, resulting in ionization.
Q3. What is radioactivity? Explain any three applications of radioactive isotopes.
The process of emission of radiation by radioactive isotopes is called radioactivity, which is a physical property of atoms. Isotopes of an element that have an unstable nucleus and can release energy in the form of radiation are called radioactive isotopes.
Example: ${^{3}_{1}H}$, ${^{14}_{6}C}$ and ${^{238}_{92}U}$.
Applications of Radioactive Isotopes:
Medical Imaging:
Technetium-99m is used in medical imaging for organs like brain, lungs, etc. Patients are injected with a small amount of this radioactive isotope, and then a special camera is used to monitor the movement of the radioactive fluid.
Carbon Dating:
Radioactive isotope C-14 is used to determine the age of carbon containing materials. The age of a sample (bone, wood, etc.) is measured by measuring the proportion of C-14. The older the sample, the less C-14 is detected.
Ionization by a Radioactive Source:
Radioactive isotopes are also used to ionize atoms. For example, radium-226 can ionize atoms through its radiation. This radiation has enough energy to remove the tightly bound electron from the orbit of its atom. This process converts that atom into a cation (positive ion).
Q4. Find out the relative atomic mass of mercury element (Hg) from the following data.
Relative Isotopic Mass
Isotopic Abundance
196Hg
0.0146%
198Hg
10.02%
199Hg
16.34 %
200Hg
23.13%
201Hg
13.22%
202Hg
29.80%
204Hg
6.85%
$
\begin{aligned}
\text{Relative atomic mass of Hg} &= \frac{(196 \times 0.0146) + (198 \times 10.02) + (199 \times 16.34)}{100} \\
&\quad + \frac{(200 \times 23.13) + (201 \times 13.22) + (202 \times 29.80) + (204 \times 6.85)}{100} \\[10pt]
&= \frac{2.8616 + 1983.96 + 3251.66}{100} \\
&\quad + \frac{4626 + 2667.22 + 6019.6 + 1403.4}{100} \\[10pt]
&= \frac{5238.4816}{100} + \frac{14716.22}{100} \\
&= 52.384816 + 147.1622 \\
&= \boxed{199.55} \quad \text{(Relative atomic mass of mercury)}
\end{aligned}
$
5. Investigative Questions
Q1. How can scientists synthesize elements in the laboratory?
Elements are synthesized through a special type of reaction called nuclear reactions, which involve nucleons (protons and neutrons). There are two major types of nuclear reactions: fission and fusion.
Fission:
Fission is a nuclear reaction in which a large nucleus is bombarded with neutrons, causing it to break into smaller nuclei. For example, in nuclear power plants, uranium-235 is bombarded with neutrons, resulting in the formation of barium (Ba) and krypton (Kr), along with the release of a large amount of energy.
$\ce{^{235}_{92}U + ^{1}_{0}n -> ^{141}_{56}Ba + ^{92}_{36}Kr + 3^{1}_{0}n + \text{Energy}}$
Fusion:
Fusion reactions, on the other hand, involve the joining of two smaller nuclei to form a new, larger nucleus. For example, when two hydrogen nuclei (protons) are forced together under extreme pressure and temperature, they form a helium nucleus and release an enormous amount of energy.
$\ce{^{2}_{1}H + ^{3}_{1}H -> ^{4}_{2}He + ^{1}_{0}n + \text{energy}}$
Q2. A system just like our solar system exists in an atom. Comment on this statement.
In the 20th century, when scientists like Rutherford and Bohr were making discoveries about the structure of the atom, they noticed a strong resemblance to our solar system. Based on this similarity, they concluded that a system similar to the solar system exists within an atom. This conclusion was drawn from the following comparisons:
Solar System
Atomic Structure
Solar system contains a very large body, sun, at its center.
An atom also contains a very large nucleus at its center.
The sun attracts the planets through gravitational force.
Nucleus attracts its surrounding electrons through electrostatic force.
Planets always revolve around the sun in their fixed orbits.
Electrons also revolve around the nucleus in fixed orbits.
As planets go farther away from the sun, the gravitational force becomes weaker.
Electrons farther from the nucleus also feel very weak attractive force.
However, it was later discovered that this resemblance is completely inaccurate, because an atom does not behave like our solar system. The solar system is governed by classical mechanics, whereas the atom is governed by quantum mechanics.