9.2.5 Bohr's Atomic Model
This is the fifth lecture from Chapter 2: ‘Atomic Structure’ of the new book for Class 9 Chemistry (Punjab Board – PCTB). It covers Niels Bohr’s atomic model. The lecture includes a multiple-choice quiz, short-answer questions, and detailed long-answer notes.
MCQs Based Quiz
Short Questions
Q1. How would you define shells, orbits and energy levels?
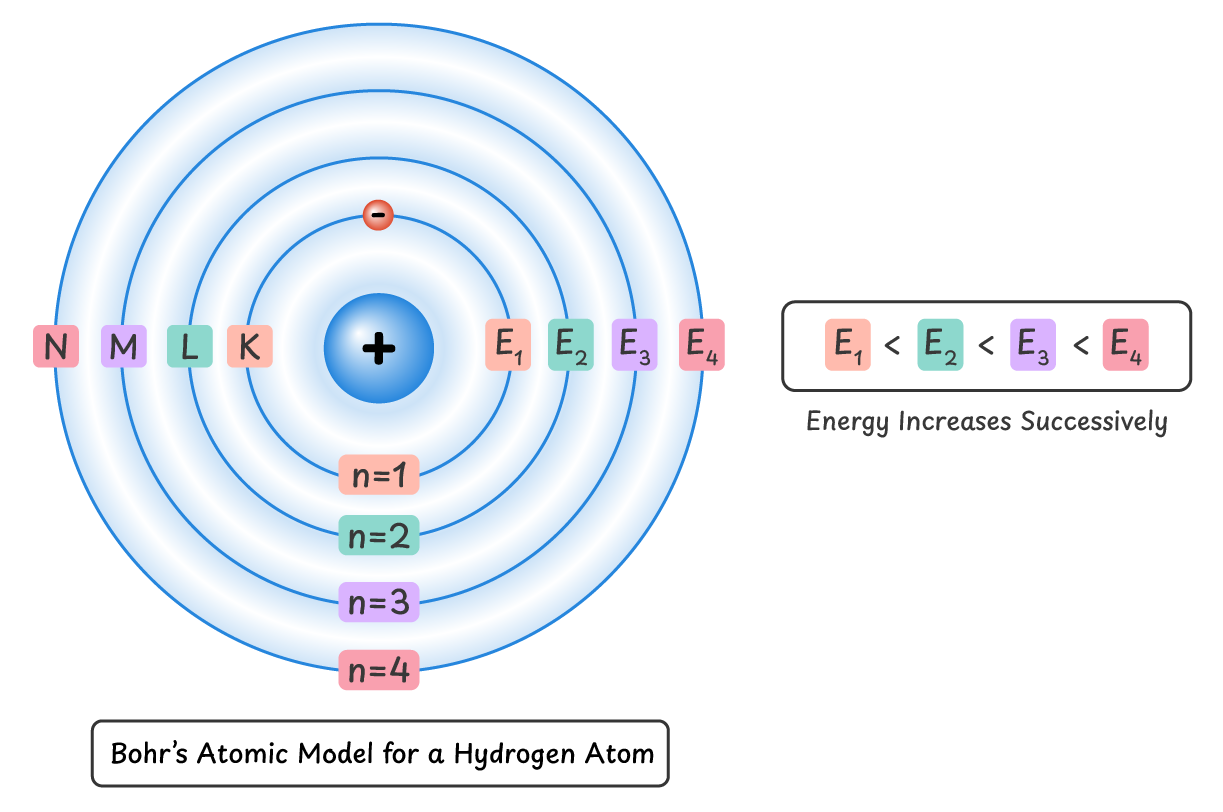
A shell, orbit, or energy level is a fixed path in which an electron revolves around the nucleus. All shells are at specific distances from the nucleus, and the energy of an electron in each shell remains constant.
Q2. How does energy change as electrons move to shells farther from the nucleus?
As an electron moves farther from the nucleus, its energy increases successively.
$E_1 < E_2 < E_3 < E_4$
Q3. What key assumption did Bohr make about electron’s energy in orbits?
- Electrons can only revolve around the nucleus in fixed energy levels.
- The first shell has the lowest energy.
- Energy increases successively as the electron moves to farther shells.
Q4. Can an electron exist between 1st and 2nd shell.
No, electrons cannot exist between shells. The space between them is called the forbidden region, and electrons are not allowed there.
Q5. Define “ground state” in Bohr’s model.
The state of an atom where all of its electrons are in the lowest possible energy levels is called ground state of that atom.
Q6. How many electrons can be accommodated in each of the first four shells?
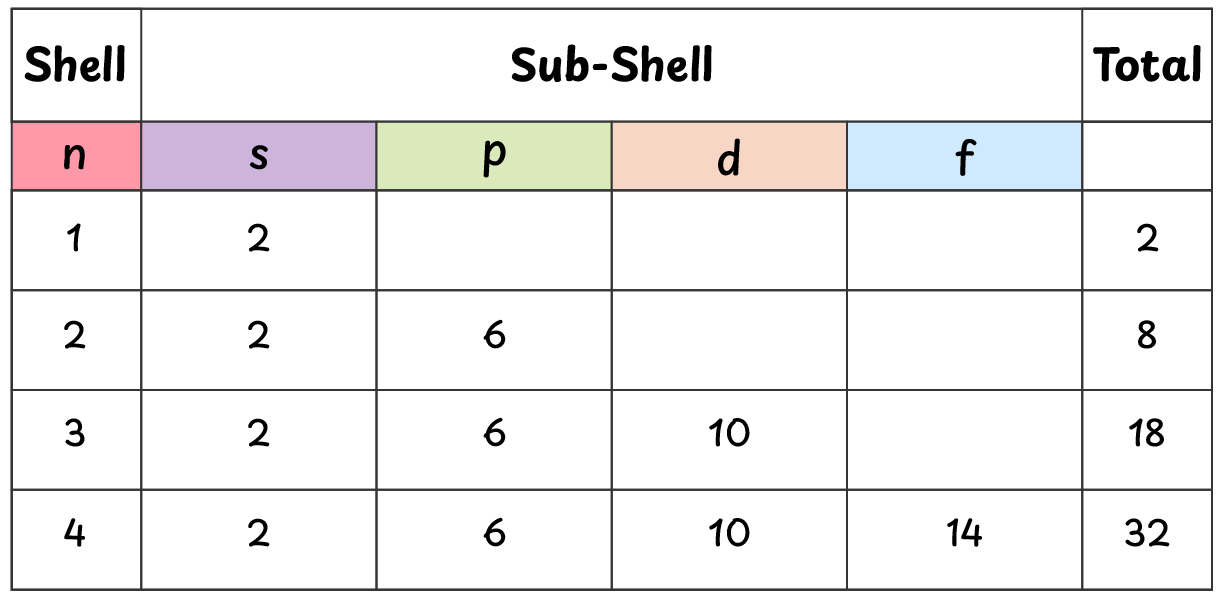
We can calculate the number of electrons in these shells using $2n^2$ formula.
First Shell (\(n=1\)):
\[ 2n^2 = 2(1)^2 = 2 \text{ electrons} \]
Second Shell (\(n=2\)):
\[ 2n^2 = 2(2)^2 = 8 \text{ electrons} \]
Third Shell (\(n=3\)):
\[ 2n^2 = 2(3)^2 = 18 \text{ electrons} \]
Fourth Shell (\(n=4\)):
\[ 2n^2 = 2(4)^2 = 32 \text{ electrons} \]
Q7. What is a sub-shell?
Each shell (energy level) is divided into smaller regions called sub-shells.
For example, 2nd shell is divided into two subshells: s and p.
Q8. Which sub-shells exist in the third shell (n=1), and what are their electron capacities?
First shell (n=1) has only one sub-shell, the s-subshell, which can accommodate 2 electrons.
Q9. Which sub-shells exist in the third shell (n=2), and what are their electron capacities?
Second shell (n=2) has two subshells: s and p.
- s-subshell can accommodate 2 electrons
- p-subshell can accommodate 6 electrons
Q10. Which sub-shells exist in the third shell (n=3), and what are their electron capacities?
Third shell (n=3) has three subshells: s, p and d.
- s-subshell can accommodate 2 electrons
- p-subshell can accommodate 6 electrons
- d-subshell can accommodate 10 electrons
Q11. Which sub-shells exist in the fourth shell (n=4), and what are their electron capacities?
Fourth shell (n=4) has four subshells: s, p, d and f.
- s-subshell can accommodate 2 electrons
- p-subshell can accommodate 6 electrons
- d-subshell can accommodate 10 electrons
- f-subshell can accommodate 14 electrons
Q12. How does Bohr’s atomic model differ from the modern atomic model regarding the nature of electrons.
Bohr’s atomic model considers electrons as particles whose location can be estimated with 100% accuracy. However, in modern atomic model, electrons are treated as negatively charged cloud and their locations can not be estimated with 100% accuracy.
Q13. What is the modern approach regarding electrons?
According to the modern approach, an electron is considered a charged cloud whose location around the nucleus cannot be predicted with 100% accuracy. Instead, its position is estimated based on the probability of finding it at a certain distance from the nucleus.
Q14. Is it possible to see an atom with our naked eyes?
No, the size of an atom is extremely small and we cannot see it with our naked eyes. An atom can only be seen using a transmission electron microscope.
Descriptive Question
Q1. Write a detailed note on Bohr’s Atomic Model.
Postulates of Bohr’s Atomic Model:
In 1913, Niels Bohr proposed an atomic model for hydrogen atom which is called Bohr’s atomic model. The model has the following postulates:
- Electrons can only revolve around the nucleus in specific paths called shells, orbits or energy levels.
- They cannot occupy any space in between these orbits.
- An electron revolving in one of these orbits has fixed energy.
- Because electrons in these orbits have fixed energy, they are also called energy levels.
- The orbit closest to the nucleus has the minimum energy.
- As the distance from the nucleus increases, the energy of the orbits also increases.
- The state of an atom when its electrons are closest to the nucleus is called the ground state.
Orbits/Shells:
The paths in which electrons revolve around the nucleus are called orbits or shells. Electrons in these shells have a fixed value of energy. Therefore, these shells are also called energy levels.
Shells that are closest to the nucleus have the minimum energy. The shells that are farther from the nucleus have successively greater energy. Electrons can only occupy these allowed energy levels.
These shells can be denoted by capital letters (K, L, M, N…) or by numbers (n = 1, 2, 3, 4…). To calculate the total number of electrons a shell can accommodate, we can use the formula $2n^2$ where n is the number of shell (1, 2, 3 …).
Subshells and Orbitals:
Shells are sub-divided into smaller energy levels called sub-shells, which can be further divided into orbitals. The number of sub-shells present in a shell is equal to its value of ‘n’. These sub-shells are also denoted by letters s, p, d and f. Electrons will first occupy lower energy levels and then fill the higher energy levels.
Modern Approach to Electrons:
In the modern atomic model, electrons are treated as charged clouds whose exact location around the nucleus cannot be predicted with 100% certainty. We can only estimate the probability of finding an electron at a certain distance from the nucleus.