9.1.7 Formation of Unsaturated and Saturated Solutions & Effect of Temperature on the Solubility of Solutes
This is the seventh lecture from Chapter 1: ‘States of Matter and Phase Changes’ of 9th Class Chemistry (Punjab Board – PCTB). It discusses the formation of unsaturated and saturated solutions. In the second part of this lecture, the effect of temperature on the solubility of solutes is also discussed — specifically, how temperature changes can affect the solubility of certain solutes. The lecture includes a multiple-choice quiz, short-answer questions, and detailed long-answer notes.
MCQs Based Quiz
Short Questions
Q1. How can you prepare a saturated solution of a solute.
To make a saturated solution, we need to keep adding the solute to the solvent until no more solute can dissolve in it.
Q2. Why is the solubility of sugar in water greater than that of salt?
Sugar have large molecules, therefore, more water molecules can surround them. Salt has smaller ions, so fewer water molecules surround them. Because more water molecules surround sugar, it dissolves more easily than salt.
Q3. What is the definition of a saturated solution?
A saturated solution is a solution in which no more solute can dissolve at a given temperature.
Q4. What is an unsaturated solution?
An unsaturated solution is a solution that can still dissolve more solute at a given temperature.
Q5. Why must we maintain the temperature of solutions while studying the solubility of different compounds?
Because the solubility changes with temperature. To compare solubility correctly, we need to keep the temperature the same.
Q6. Write three compounds whose solubility increases with increase in temperature.
- KCl (Potassium Chloride)
- KNO3 (Potassium Nitrate
- AgNO3 (Silver Nitrate)
Q7. Write three compounds whose solubility has an inverse relation with temperature.
- HCl (Hydrogen Chloride)
- NH3 (Ammonia)
- CO2 (Carbon Dioxide)
Q8. Do you know any compound whose solubility is not affected by the variations in temperature?
Table Salt (NaCl) is a compound whose solubility is not affected by the variations in temperature.
Q9. How variations of solubility at different temperatures can be useful to use?
- We can use variations in the solubility of solutes to purify them through a process called crystallization.
- By understanding this, we can also store and preserve food and medicines more efficiently.
Descriptive Questions
Q1. How would you describe the formation of unsaturated solutions and saturated solutions?
Unsaturated Solution:
A solution that can dissolve more solute at a particular temperature is called an unsaturated solution.
Saturated Solution:
A solution which cannot dissolve any more solute at a particular temperature is called a saturated solution.
Example:
If you take 100g of water and begin adding table sugar in small amounts, the sugar will keep dissolving. You can continue adding sugar until it no longer dissolves in the water.
- When the solution can still dissolve more sugar, it is called an unsaturated solution.
- When no more sugar dissolves and starts settling at the bottom, it becomes a saturated solution.
Solubility:
The maximum amount of solute that can be dissolved in a 100g solvent at a particular temperature is called the solubility of that solute.
Example:
At 20°C in 100g of water:
- Solubility of table salt (NaCl) = 36g
- Solubility of table sugar = 203.9g
Different solutes have different solubilities in a solvent. The solubility of a solute depends on the following factors:
- Temperature of the solution
- Size of solute particles
Size of Solute Particles and Effect on Solubility:
Table sugar has higher solubility than table salt in water. This is because:
- Sugar molecules are larger than salt ions.
- More water molecules can surround sugar molecules due to their large size.
Due to large size of sugar (solute) molecules more water (solvent) molecules surround it. This leads to the better solubility of sugar in water as compared to salt.
Q2. Explain the effects of temperature on the solubility of solutes. What are some applications of the variation in the solubility of solutes?
Solubility:
Solubility is the amount of solute that can be dissolved in 100g of a solvent at a specific temperature.
Types of solutes:
Temperature has different effects on the solubility of the solutes. Based on this, solutes can be classified into three main categories:
Solutes with a direct relationship to temperature:
The solubility of these solutes increases with the increase in temperature.
Example: KNO3, KCl, NaNO3
Solutes with an inverse relationship to temperature
The solubility of these solutes increases with the decrease in temperature.
Example: HCl, CO2, NH₃
Solutes unaffected by temperature
These solutes show little or no change in solubility with temperature variations.
Example: NaCl
Solubility curves of the solutes:
The solubility of solutes at different temperatures can be understood by studying their solubility curves.
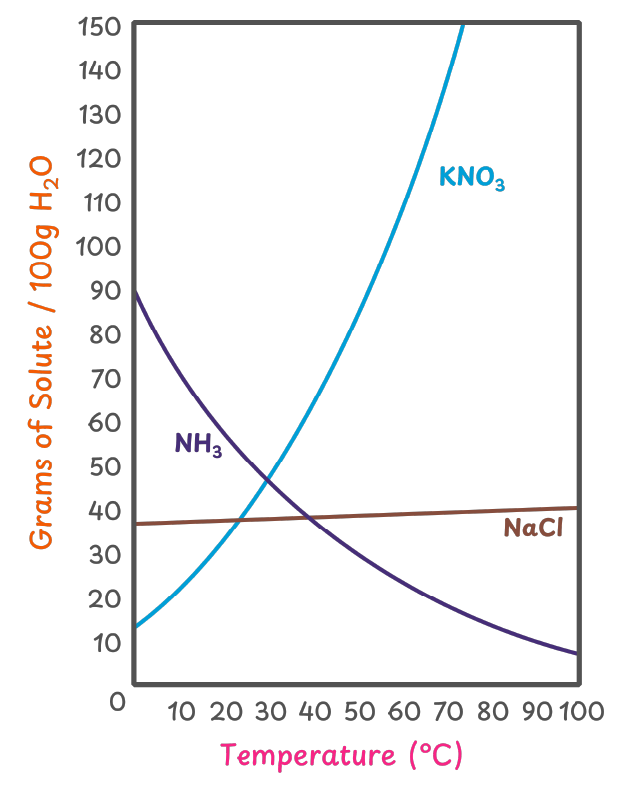
Applications of Variations of Solubility of the Solute:
- We can use the variations in solubility of the solutes to purify them. Solubility of solutes like KNO3 increases with temperature. We can use this to purify them through a process called crystallization.
- The solubility of gases like CO2 decreases with increase in temperature. By knowing this we store soda bottles at colder temperatures to preserve their taste.